
PDF Publication Title:
Text from PDF Page: 009
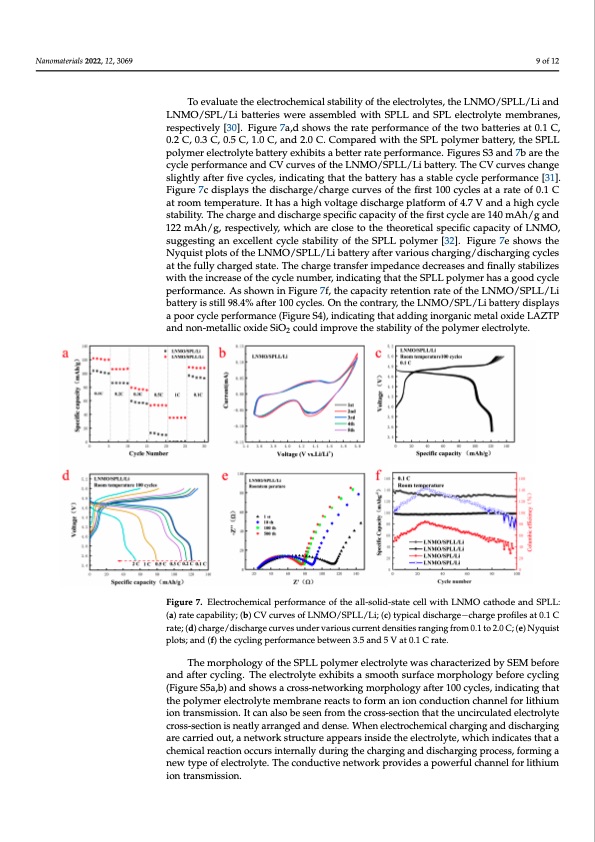
Nanomaterials 2022, 12, 3069 9 of 12 To evaluate the electrochemical stability of the electrolytes, the LNMO/SPLL/Li and LNMO/SPL/Li batteries were assembled with SPLL and SPL electrolyte membranes, respectively [30]. Figure 7a,d shows the rate performance of the two batteries at 0.1 C, 0.2 C, 0.3 C, 0.5 C, 1.0 C, and 2.0 C. Compared with the SPL polymer battery, the SPLL polymer electrolyte battery exhibits a better rate performance. Figures S3 and 7b are the cycle performance and CV curves of the LNMO/SPLL/Li battery. The CV curves change slightly after five cycles, indicating that the battery has a stable cycle performance [31]. Figure 7c displays the discharge/charge curves of the first 100 cycles at a rate of 0.1 C at room temperature. It has a high voltage discharge platform of 4.7 V and a high cycle stability. The charge and discharge specific capacity of the first cycle are 140 mAh/g and 122 mAh/g, respectively, which are close to the theoretical specific capacity of LNMO, suggesting an excellent cycle stability of the SPLL polymer [32]. Figure 7e shows the Nyquist plots of the LNMO/SPLL/Li battery after various charging/discharging cycles at the fully charged state. The charge transfer impedance decreases and finally stabilizes with the increase of the cycle number, indicating that the SPLL polymer has a good cycle materials 2022, 12, x FOR PEER REVIEW performance. As shown in Figure 7f, the capacity retention rate of the LNMO/SPLL/Li battery is still 98.4% after 100 cycles. On the contrary, the LNMO/SPL/Li battery displays a poor cycle performance (Figure S4), indicating that adding inorganic metal oxide LAZTP and non-metallic oxide SiO2 could improve the stability of the polymer electrolyte. Figure 7. Electrochemical performance of the all-solid-state cell with LNMO cathode and SPLL: (a) rate capability; (b) CV curves of LNMO/SPLL/Li; (c) typical discharge−charge profiles at 0.1 C Figure 7. Electrochemical performance of the all-solid-state cell with LNMO cathode and S rate; (d) charge/discharge curves under various current densities ranging from 0.1 to 2.0 C; (e) Nyquist rate cpaloptsa;bainldity(f;) t(hbe)cCycVlincguprevrfeosrmoafnLceNbMetwOe/eSnP3L.5La/nLdi;5(Vc)aty0.p1iCcaraltde.ischarge−charge profiles at 0. (d) charge/discharge curves under various current densities ranging from 0.1 to 2.0 C; (e) The morphology of the SPLL polymer electrolyte was characterized by SEM before plots; and (f) the cycling performance between 3.5 and 5 V at 0.1 C rate. and after cycling. The electrolyte exhibits a smooth surface morphology before cycling (Figure S5a,b) and shows a cross-networking morphology after 100 cycles, indicating that tThehpeomlymorepr ehloecltoroglyteomf tehmebrSaPnLe rLeapctosltyomfoermr ealneciotnrocloyntdeuwctiaons chaanrnaecl tfeor ilzitehdiumby SEM ion transmission. It can also be seen from the cross-section that the uncirculated electrolyte and after cycling. The electrolyte exhibits a smooth surface morphology before cross-section is neatly arranged and dense. When electrochemical charging and discharging (Figure S5a,b) and shows a cross-networking morphology after 100 cycles, indicati are carried out, a network structure appears inside the electrolyte, which indicates that a the polymer electrolyte membrane reacts to form an ion conduction channel for chemical reaction occurs internally during the charging and discharging process, forming a ion tnraewnstmypiesosfioelnec.tIrtoclyatne. aTlhseocbonedsuecetinvefrnoetmwotrhkepcrorvoisdse-ssaecptoiwonerftuhlacthtahnneeul fnocrilritchuiulamted ele ion transmission. cross-section is neatly arranged and dense. When electrochemical charging and di ing are carried out, a network structure appears inside the electrolyte, which indica a chemical reaction occurs internally during the charging and discharging process ing a new type of electrolyte. The conductive network provides a powerful cha o1 lithium ion transmission. P 1 N c n l c s t nPDF Image | Simple Three-Matrix Solid Electrolyte Membrane in Air
PDF Search Title:
Simple Three-Matrix Solid Electrolyte Membrane in AirOriginal File Name Searched:
nanomaterials-12-03069.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |