
PDF Publication Title:
Text from PDF Page: 008
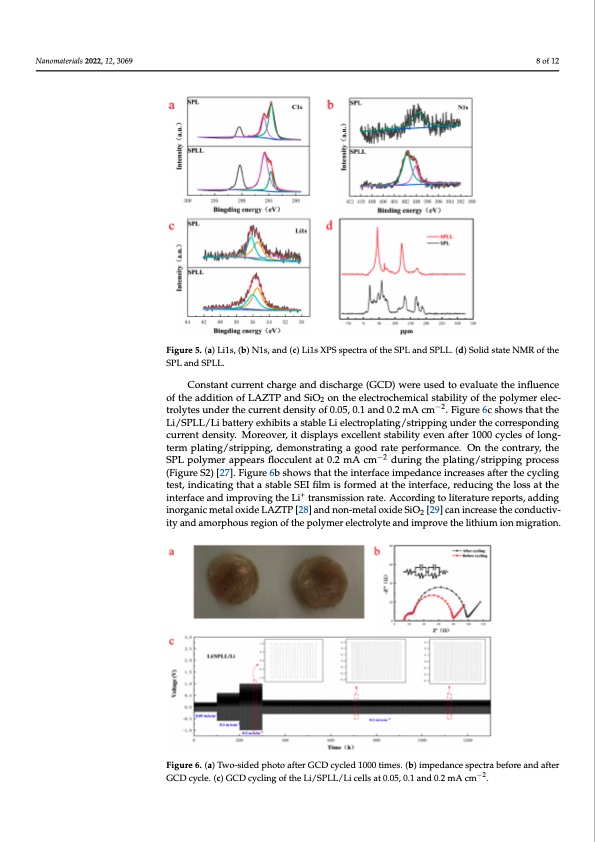
electrolyte and provides a lithium ion migration channel, we analyzed the local chemical s - d e f - e g - L e - e c d Nanomaterials 2022, 12, 3069 environment of lithium ion by 13C solid-state nuclear magnetic resonance (NMR). A shown in Figure 5d, the peaks of SPL are scattered, and there are many impurity peaks In contrast, for SPLL, the peaks near 50 ppm and 140 ppm gradually become sharp, indi cating that the addition of LAZTP and SiO2 reduces the crystallinity of SPLL polymer an enhances the conductivity of the material, which is consistent with the EIS results [26]. SPL and SPLL. SPL and SPLL. Constant current charge and discharge (GCD) were used to evaluate the influence of the addition of LAZTP and SiO on the electrochemical stability of the polymer elec- Constant current charge an2d discharge (GCD) were used to evaluate the influence o trolytes under the current density of 0.05, 0.1 and 0.2 mA cm−2. Figure 6c shows that the the addition of LAZTP and SiO2 on the electrochemical stability of the polymer electro Li/SPLL/Li battery exhibits a stable Li electroplating/stripping under the corresponding lytes under the current density of 0.05, 0.1 and 0.2 mA cm−2. Figure 6c shows that th 8 of 12 Figure 5. (a) Li1s, (b) N1s, and (c) Li1s XPS spectra of the SPL and SPLL. (d) Solid state NMR of the Figure 5. (a) Li1s, (b) N1s, and (c) Li1s XPS spectra of the SPL and SPLL. (d) Solid state NMR of th current density. Moreover, it displays excellent stability even after 1000 cycles of long- Li/SPLL/Li battery exhibits a stable Li electroplating/stripping under the correspondin term plating/stripping, demonstrating a good rate performance. On the contrary, the current density. Moreover, it displays exce−ll2ent stability even after 1000 cycles of long SPL polymer appears flocculent at 0.2 mA cm during the plating/stripping process term plating/stripping, demonstrating a good rate performance. On the contrary, the SP (Figure S2) [27]. Figure 6b shows that the interface impedance increases after the cycling polymer appears flocculent at 0.2 mA cm−2 during the plating/stripping process (Figur test, indicating that a stable SEI film is formed at the interface, reducing the loss at the Nanomaterials 2022, 12, x FOR PEER REVIEW + 9 of 13 Sin2t)er[f2a7c]e. aFnigduimrep6robvsinhgowthse Lthi atrtahnesminitsesirofancreatiem. Apeccdoarndcinegintocrlietaersaetsuraeftrerpothrtes,caydcdling test, in inorganicmetaloxideLAZTP[28]andnon-metaloxideSiO [29]canincreasetheconductiv- dicating that a stable SEI film is formed at the interfa2ce, reducing the loss at the interfac ity and amorphous regio+n of the polymer electrolyte and improve the lithium ion migration. and improving the Li transmission rate. According to literature reports, adding inorgani metal oxide LAZTP [28]and non-metal oxide SiO2 [29] can increase the conductivity an amorphous region of the polymer electrolyte and improve the lithium ion migration. Figure 6. (a) Two-sided photo after GCD cycled 1000 times. (b) impedance spectra before and after Figure 6. (a) Two-sided photo after GCD cycled 1000 times. (b) impedance spectra before and after GCD cycle. (c) GCD cycling of the Li/SPLL/Li cells at 0.05, 0.1 and 0.2 mA cm−2. GCD cycle. (c) GCD cycling of the Li/SPLL/Li cells at 0.05, 0.1 and 0.2 mA cm−2. To evaluate the electrochemical stability of the electrolytes, the LNMO/SPLL/Li and LNMO/SPL/Li batteries were assembled with SPLL and SPL electrolyte membranes, re- spectively [30]. Figure 7a,d shows the rate performance of the two batteries at 0.1 C, 0.2 C,PDF Image | Simple Three-Matrix Solid Electrolyte Membrane in Air
PDF Search Title:
Simple Three-Matrix Solid Electrolyte Membrane in AirOriginal File Name Searched:
nanomaterials-12-03069.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |