
PDF Publication Title:
Text from PDF Page: 007
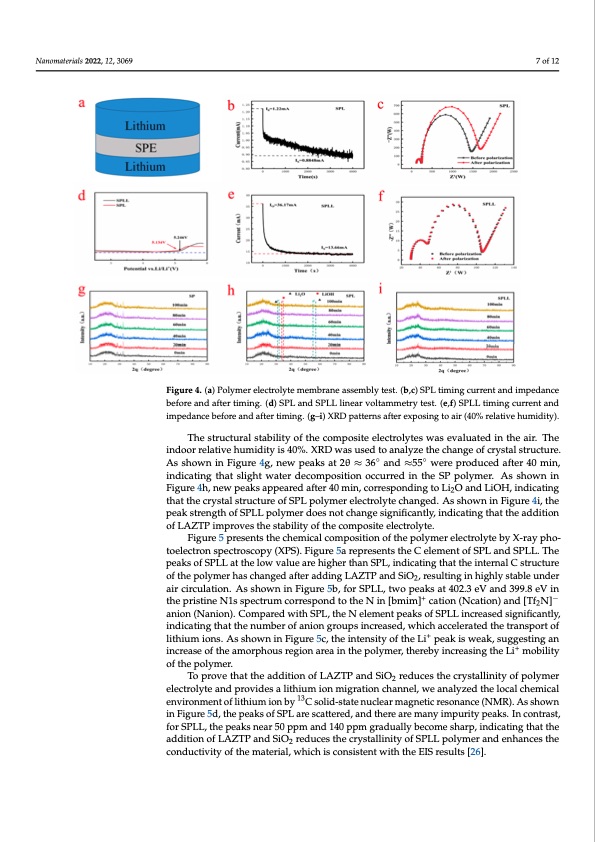
indicating that slight water decomposition occurred in the SP polymer. As shown in Fig ure 4h, new peaks appeared after 40 min, corresponding to Li2O and LiOH, indicating tha the crystal structure of SPL polymer electrolyte changed. As shown in Figure 4i, the pea strength of SPLL polymer does not change significantly, indicating that the addition o Nanomaterials 2022, 12, 3069 7 of 12 LAZTP improves the stability of the composite electrolyte. Figure 4. (a) Polymer electrolyte membrane assembly test. (b,c) SPL timing current and impedance Figure 4. (a) Polymer electrolyte membrane assembly test. (b,c) SPL timing current and impedanc before and after timing. (d) SPL and SPLL linear voltammetry test. (e,f) SPLL timing current and before and after timing. (d) SPL and SPLL linear voltammetry test. (e,f) SPLL timing current an impedance before and after timing. (g–i) XRD patterns after exposing to air (40% relative humidity). impedance before and after timing. (g–i) XRD patterns after exposing to air (40% relative humidity) The structural stability of the composite electrolytes was evaluated in the air. The Figure 5 presents the chemical composition of the polymer electrolyte by X-ray pho indoor relative humidity is 40%. XRD was used to analyze the change of crystal structure. ◦◦ toelectroAns shpoewcntroinscFoigpuyre(4XgP, Sn)e.wFipgeuakres a5ta2θre≈pr3e6seanntds t≈h5e5 CweelremperondtuocfedSPafLteran40dmSiPnL, L. Th indicating that slight water decomposition occurred in the SP polymer. As shown in peaks of SPLL at the low value are higher than SPL, indicating that the internal C structur Figure 4h, new peaks appeared after 40 min, corresponding to Li2O and LiOH, indicating of the polymer has changed after adding LAZTP and SiO2, resulting in highly stable unde that the crystal structure of SPL polymer electrolyte changed. As shown in Figure 4i, the air circulation. As shown in Figure 5b, for SPLL, two peaks at 402.3 eV and 399.8 eV in th peak strength of SPLL polymer does not change significantly, indicating that the addition +− pristineoNfL1AsZsTpPecimtrpurmovecsotrhreestpaboinlitdytoofttheecoNmipnos[ibtemeliemct]rolcyateti.on(Ncation)and[Tf2N] anio Figure 5 presents the chemical composition of the polymer electrolyte by X-ray pho- toelectron spectroscopy (XPS). Figure 5a represents the C element of SPL and SPLL. The peaks of SPLL at the low value are higher than SPL, indicating that the internal C structure of the polymer has changed after adding LAZTP and SiO2, resulting in highly stable under air circulation. As shown in Figure 5b, for SPLL, two peaks at 402.3 eV and 399.8 eV in the pristine N1s spectrum correspond to the N in [bmim]+ cation (Ncation) and [Tf2N]− anion (Nanion). Compared with SPL, the N element peaks of SPLL increased significantly, indicating that the number of anion groups increased, which accelerated the transport of lithium ions. As shown in Figure 5c, the intensity of the Li+ peak is weak, suggesting an increase of the amorphous region area in the polymer, thereby increasing the Li+ mobility of the polymer. To prove that the addition of LAZTP and SiO2 reduces the crystallinity of polymer electrolyte and provides a lithium ion migration channel, we analyzed the local chemical environment of lithium ion by 13C solid-state nuclear magnetic resonance (NMR). As shown in Figure 5d, the peaks of SPL are scattered, and there are many impurity peaks. In contrast, for SPLL, the peaks near 50 ppm and 140 ppm gradually become sharp, indicating that the addition of LAZTP and SiO2 reduces the crystallinity of SPLL polymer and enhances the conductivity of the material, which is consistent with the EIS results [26]. - t k f e d . - e e r e nPDF Image | Simple Three-Matrix Solid Electrolyte Membrane in Air
PDF Search Title:
Simple Three-Matrix Solid Electrolyte Membrane in AirOriginal File Name Searched:
nanomaterials-12-03069.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |