
PDF Publication Title:
Text from PDF Page: 008
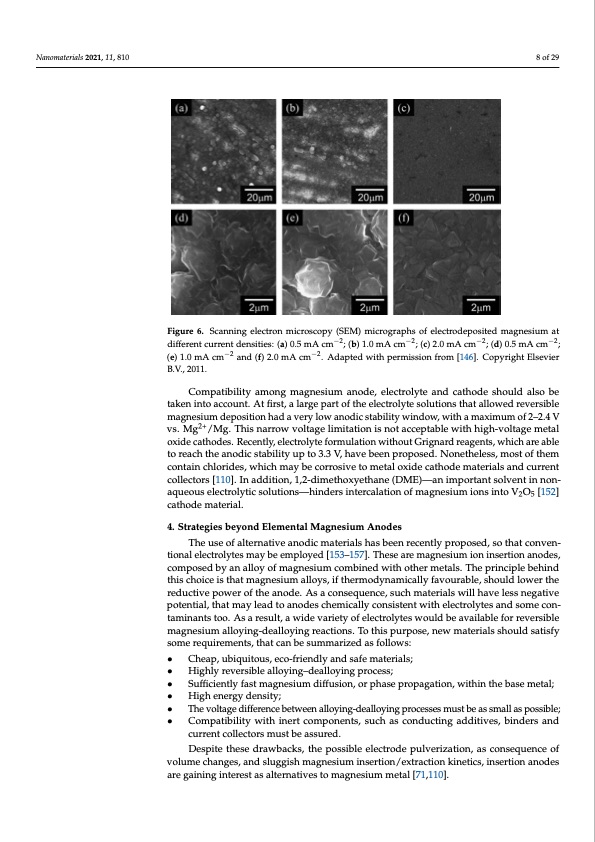
Nanomaterials 2021, 11, 810 hypothesize that both thermodynamic stability and diffusion rates of Mg crystals growth of magnesium depositions [71,146]. FFigiugrue r6e. 6Sc.aSncnaingneinlegctreolnecmtircoronscmopiycr(oSEsMco)pmyic(rSogErMap)hsmoifcerloecgtroadpehpossiotefdemleacgtnroesdiuempoast ited m but, as shown in Figure 6, there is a preferred orientation of the deposits the current density. For instance, (001) orientation is the preferred one at l sities, while the (100) is preferred at high current densities. Consequently, 1.0 mA cm−2 and (f) 2.0 mA cm−2. Adapted with permission from [146]. Copyright B.V., 2011. 2011. Compatibility among magnesium anode, electrolyte and cathode should also be taken into account. At first, a large part of the electrolyte solutions that allowed reversible It is expected that advancements in the understanding of magnesium magnesium deposition had a very low anodic stability window, with a maximum of 2–2.4 V lead 2t+o the achievement of advanced SEI layers, similar to those formed in vs. Mg /Mg. This narrow voltage limitation is not acceptable with high-voltage metal o[x1id4e7c–a1th5o1d]e.s.TRoecdenatltye,,elietctirsoloytfecforrumcuialaltioimn wpiothrotuatnGcreigntharadtretahgenatsn, owdhiechraerme aabilens free to reach the anodic stability up to 3.3 V, have been proposed. Nonetheless, most of them contain chlorides, which may be corrosive to metal oxide cathode materials and current collectors [110]. In addition, 1,2-dimethoxyethane (DME)—an important solvent in non- aqueous electrolytic solutions—hinders intercalation of magnesium ions into V2O5 [152] cathode material. 4. Strategies beyond Elemental Magnesium Anodes The use of alternative anodic materials has been recently proposed, so that conven- tional electrolytes may be employed [153–157]. These are magnesium ion insertion anodes, composed by an alloy of magnesium combined with other metals. The principle behind this choice is that magnesium alloys, if thermodynamically favourable, should lower the reductive power of the anode. As a consequence, such materials will have less negative potential, that may lead to anodes chemically consistent with electrolytes and some con- taminants too. As a result, a wide variety of electrolytes would be available for reversible magnesium alloying-dealloying reactions. To this purpose, new materials should satisfy some requirements, that can be summarized as follows: • Cheap, ubiquitous, eco-friendly and safe materials; • Highly reversible alloying–dealloying process; • Sufficiently fast magnesium diffusion, or phase propagation, within the base metal; • High energy density; • The voltage difference between alloying-dealloying processes must be as small as possible; • Compatibility with inert components, such as conducting additives, binders and current collectors must be assured. Despite these drawbacks, the possible electrode pulverization, as consequence of volume changes, and sluggish magnesium insertion/extraction kinetics, insertion anodes are gaining interest as alternatives to magnesium metal [71,110]. 8 of 29 −2 −2 −2 −2 different current densities: (a) 0.5 mA cm ; (b) 1.0 m−A2 cm ; (c) 2.0 mA cm−2 ; (d) 0.5 mA cm −2; differentcurrentdensities:(a)0.5mAcm ;(b)1.0mAcm ;(c)2.0mAcm ;(d)0. (e) 1.0 mA cm−2 and (f) 2.0 mA cm−2. Adapted with permission from [146]. Copyright Elsevier , o i a 5 E tPDF Image | Overview on Anodes for Magnesium Batteries
PDF Search Title:
Overview on Anodes for Magnesium BatteriesOriginal File Name Searched:
nanomaterials-11-00810.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |