
PDF Publication Title:
Text from PDF Page: 036
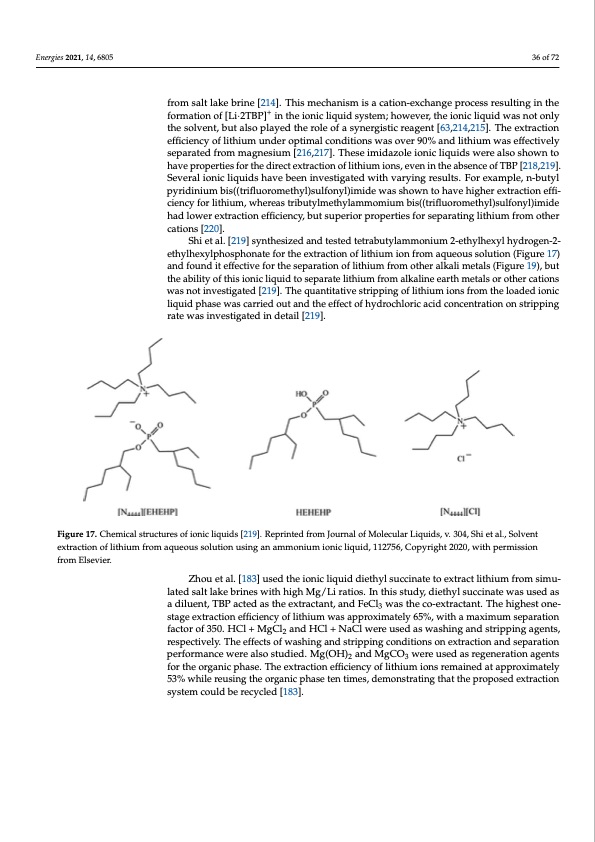
Energies 2021, 14, 6805 36 of 72 4, x FOR PEER REVIEW from salt lake brine [214]. This mechanism is a cation-exchange process resulting in the formation of [Li·2TBP]+ in the ionic liquid system; however, the ionic liquid was not only the solvent, but also played the role of a synergistic reagent [63,214,215]. The extraction 38 of 74 efficiency of lithium under optimal conditions was over 90% and lithium was effectively separated from magnesium [216,217]. These imidazole ionic liquids were also shown to have properties for the direct extraction of lithium ions, even in the absence of TBP [218,219]. bis((trifluoromethSyelv)esruallfoionnyicl)ilimquidideswhavsesbheoewn ninvtoesthigaavtedhwigihthervaerxytirnagctrieosnuletsf.fiFcoiernecxyamfoprle, n-butyl pyridinium bis((trifluoromethyl)sulfonyl)imide was shown to have higher extraction effi- lithium, whereas tributylmethylammomium bis((trifluoromethyl)sulfonyl)imide had ciency for lithium, whereas tributylmethylammomium bis((trifluoromethyl)sulfonyl)imide lower extraction efficiency, but superior properties for separating lithium from other cations [220]. had lower extraction efficiency, but superior properties for separating lithium from other cations [220]. Shi et al. [219] synthesized and tested tetrabutylammonium 2-ethylhexyl hydrogen- Shi et al. [219] synthesized and tested tetrabutylammonium 2-ethylhexyl hydrogen-2- 2-ethylhexylphosphonate for the extraction of lithium ion from aqueous solution (Figure ethylhexylphosphonate for the extraction of lithium ion from aqueous solution (Figure 17) 17) and found it effective for the separation of lithium from other alkali metals (Figure 19), and found it effective for the separation of lithium from other alkali metals (Figure 19), but but the ability of this ionic liquid to separate lithium from alkaline earth metals or other the ability of this ionic liquid to separate lithium from alkaline earth metals or other cations cations was not iwnavsenstoitgiantveedsti[g2a1t9e]d. [T21h9e].qTuhaenqtuitanttiivtaetisvterisptrpipinpgingofoflilitthiium iionnssfrformomthethloeaded ionic loaded ionic liquildiqpuhidaspehwasaeswcasrrciaerdrieoduotuatnadndthtehefeffefcecttooffhhydrochlorriiccaaccididcocnocnecnetnrattriaotnion stripping rate was investigated in detail [219]. on stripping rate was investigated in detail [219]. Figure 17. Chemical structures of ionic liquids [219]. Reprinted from Journal of Molecular Liquids, Figure 17. Chemical structures of ionic liquids [219]. Reprinted from Journal of Molecular Liquids, v. 304, Shi et al., Solvent v. 304, Shi et al., Solvent extraction of lithium from aqueous solution using an ammonium ionic extraction of lithium from aqueous solution using an ammonium ionic liquid, 112756, Copyright 2020, with permission liquid, 112756, Copyright 2020, with permission from Elsevier. Zhou et al. [183] used the ionic liquid diethyl succinate to extract lithium from simu- lated salt lake brines with high Mg/Li ratios. In this study, diethyl succinate was used as a diluent, TBP acted as the extractant, and FeCl3 was the co-extractant. The highest one- stage extraction efficiency of lithium was approximately 65%, with a maximum separation factor of 350. HCl + MgCl2 and HCl + NaCl were used as washing and stripping agents, respectively. The effects of washing and stripping conditions on extraction and separation performance were also studied. Mg(OH)2 and MgCO3 were used as regeneration agents for the organic phase. The extraction efficiency of lithium ions remained at approximately 53% while reusing the organic phase ten times, demonstrating that the proposed extraction system could be recycled [183]. from Elsevier.PDF Image | Recovery of Lithium from Geothermal Brines
PDF Search Title:
Recovery of Lithium from Geothermal BrinesOriginal File Name Searched:
energies-14-06805-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |