
PDF Publication Title:
Text from PDF Page: 014
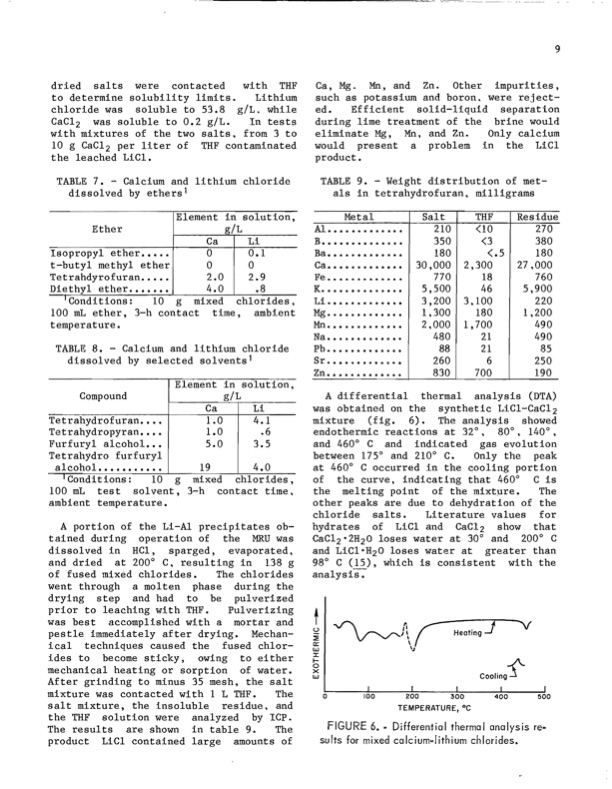
dried salts were contacted with THF to determine solubility limits. Lithium chloride was soluble to 53.8 gIL. while CaCl2 was soluble to 0.2 gIL. In tests with mixtures of the two salts, from 3 to 10 g CaCl2 per liter of THF contaminated the leached LiCI. TABLE 7. - Calcium and lithium chloride dissolved by ethers 1 Element in solution, gIL Ca, Mg. Mn, and Zn. Other impurities, such as potassium and boron. were reject- ed. Efficient solid-liquid separation during lime treatment of the brine would eliminate Mg, Mn, and Zn. Only calcium would present a problem in the LiCI product. TABLE 9. - Weight distribution of met- als in tetrahydrofuran, milligrams Residue 210 <10 270 350 <3 380 180 <.5 180 30,000 2,300 27,000 770 18 760 5,500 46 5,900 3,200 3,100 220 100 mL ether, 3-h contact time, ambient Mg......................... 1,300 180 1,200 2,000 1,700 490 480 21 490 88 21 85 260 6 250 830 700 190 Ether Isopropyl ether..... t-butyl methyl ether Tetrahdyrofuran..... 2.0 2.9 Diethyl ether....... 4.0 .8 Ba ...................... Ca ...................... Fe ........................ K....................... Li ...................... 'Conditions: 10 g mixed chlorides, temperature. Mn ....................... Na ......................... Pb 4 . . . . . . . . . . . . . . . . . . . . . . . . Sr ......................... Zn .......................... TABLE 8. - Calcium and lithium chloride dissolved by selected solvents 1 Compound Tetrahydrofuran•••• Tetrahydropyran•••• Furfuryl alcohol ••• Tetrahydro furfuryl gIL A differential thermal analysis (DTA) was obtained on the synthetic LiCI-CaCI 2 mixture (fig. 6). The analysis showed endothermic reactions at 32°, 80°, 140°, and 460° C and indicated gas evolution between 175° and 210° C. Only the peak at 460° C occurred in the cooling portion went through a molten phase during the drying step and had to be pulverized prior to leaching with THF. Pulverizing t was best accomplished with a mortar and pestle immediately after drying. Mechan- ical techniques caused the fused chlor- ides to become sticky, owing to either u ~ a;: w :x: I- Heating] V COOling-f'- mechanical heating or sorption of water. ox After grinding to minus 35 mesh, the salt mixture was contacted with 1 L THF. The salt mixture, the insoluble residue, and the THF solution were analyzed by ICP. The results are shown in table 9. The product LiCI contained large amounts of w Ca Li 0 0.1 0 0 Element in solution, Ca 1.0 1.0 5.0 Li 4.1 .6 3.5 alcohol........... 'Conditions: 10 g mixed chlorides, of the curve, indicating that 460° C is 19 4.0 100 mL test solvent, 3-h contact time, the melting point of the mixt~re. The ambient temperature. other peaks are due to dehydration of the chloride salts. Literature values for A portion of the Li-AI precipitates ob- hydrates of LiCI and CaCl2 show that tained during operation of the MRU was CaCI2'2H20 loses water at 30° and 200° C dissolved in HCI, sparged, evaporated, and LiCl'H20 loses water at greater than and dried at 200° C, resulting in 138 g 98° C (15), which is consistent with the of fused mixed chlorides. The chlorides analysiS:- Metal Al ..................... B .......................... Salt THF o FIGURE 6. ~ Djfferential thermal analysis rea suits for mixed calciumalithium chlorides. 9PDF Image | Recovering Lithium Chloride From a Geothermal Brine 1984
PDF Search Title:
Recovering Lithium Chloride From a Geothermal Brine 1984Original File Name Searched:
cdc_10654_DS1.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |