
PDF Publication Title:
Text from PDF Page: 015
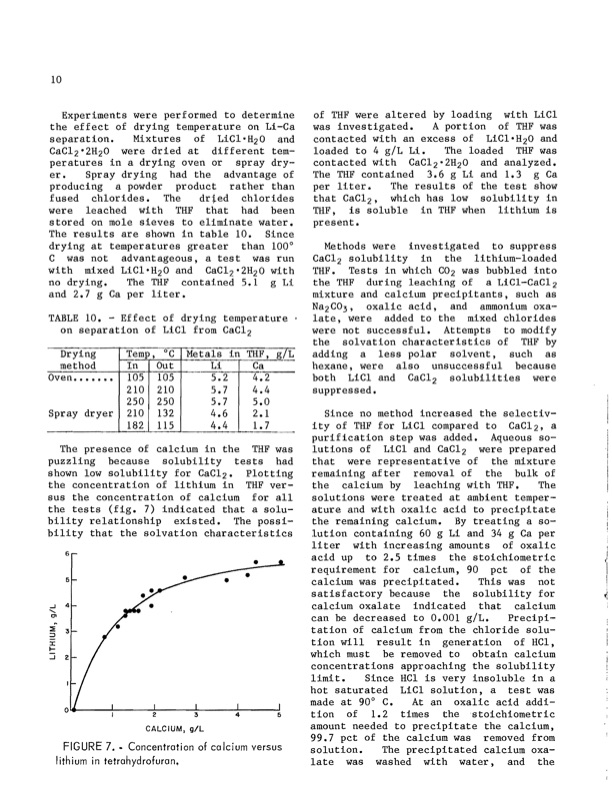
10 Experiments were performed to determine of THF were altered by loading with LiCl the effect of drying temperature on Li-Ca was investigated. A portion of THF was separation. Mixtures of LiCl·H20 and CaCl2·2H20 were dried at different tem- peratures in a drying oven or spray dry- er. Spray drying had the advantage of producing a powder product rather than fused chlorides. The dried chlorides were leached with THF that had been stored on mole sieves to eliminate water. The results are shown in table 10. Since drying at temperatures greater than 1000 C was not advantageous, a test was run with mixed LiCl·H20 and CaCl2 ·2H20 with no drying. The THF contained 5.1 g Li and 2.7 g Ca per liter. TABLE 10. - Effect of drying temperature on separation of LiCl from CaCl2 Drying Temp, °C Metals in THF, giL contacted with an excess of LiCl·H20 and loaded to 4 giL Li. The loaded THF was contacted with CaC1202H20 and analyzed. The THF contained 3.6 g Li and 1.3 g Ca per liter. The results of the test show that CaC12 , which has low solubility in THF, is soluble in THF when lithium is present. Methods were investigated to suppress CaC12 solubility in the lithium-loaded THF. Tests in which CO2 was bubbled into the THF during leaching of a LiCl-CaC12 mixture and calcium precipitants, such as Na2C03, oxalic acid, and ammonium oxa- late, were added to the mixed chlorides were not successful. Attempts to modify the solvation characteristics of THF by adding a less polar solvent, such as hexane, were also unsuccessful because both LiCl and CaC12 solubilities were suppressed. method In Out Oven...•.•• 105 105 210 210 250 250 Spray dryer 210 132 182 115 Li Ca 5.2 4.2 5.7 4.4 5.7 5.0 4.6 2.1 4.4 1.7 Since no method increased the selectiv- ity of THF for LiCl compared to CaC12, a purification step was added. Aqueous so- lutions of LiCl and CaCl2 were prepared The presence of calcium in the THF was puzzling because solubility tests had that were representative of the mixture shown low solubility for CaC12' Plotting remaining after removal of the bulk of the concentration of lithium in THF ver- the calcium by leaching with THF. The sus the concentration of calcium for all solutions were treated at ambient temper- the tests (fig. 7) indicated that a solu- ature and with oxalic acid to precipitate bility relationship existed. The possi- the remaining calcium. By treating a so- bility that the solvation characteristics 6 5 ..J 4 ...... C>1 ~ ::t 3 :::l :c I- ::::i :2 • lution containing 60 g Li and 34 g Ca per liter with increasing amounts of oxalic acid up to 2.5 times the stoichiometric requirement for calcium, 90 pct of the calcium was precipitated. This was not satisfactory because the solubility for calcium oxalate indicated that calcium can be decreased to 0.001 giL. Precipi- tation of calcium from the chloride solu- tion will result in generation of HCI, which must be removed to obtain calcium concentrations approaching the solubility limit. Since HCl is very insoluble in a hot saturated LiCl solution, a test was made at 900 C. At an oxalic acid addi- tion of 1. 2 times the stoichiometric amount needed to precipitate the calcium, 99.7 pct of the calcium was removed from solution. The precipitated calcium oxa- late was washed with water, and the CALCIUM, giL FIGURE 7•• Concentration of calcium versus lithium in tetrahydrofuran. i '1PDF Image | Recovering Lithium Chloride From a Geothermal Brine 1984
PDF Search Title:
Recovering Lithium Chloride From a Geothermal Brine 1984Original File Name Searched:
cdc_10654_DS1.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |