
PDF Publication Title:
Text from PDF Page: 016
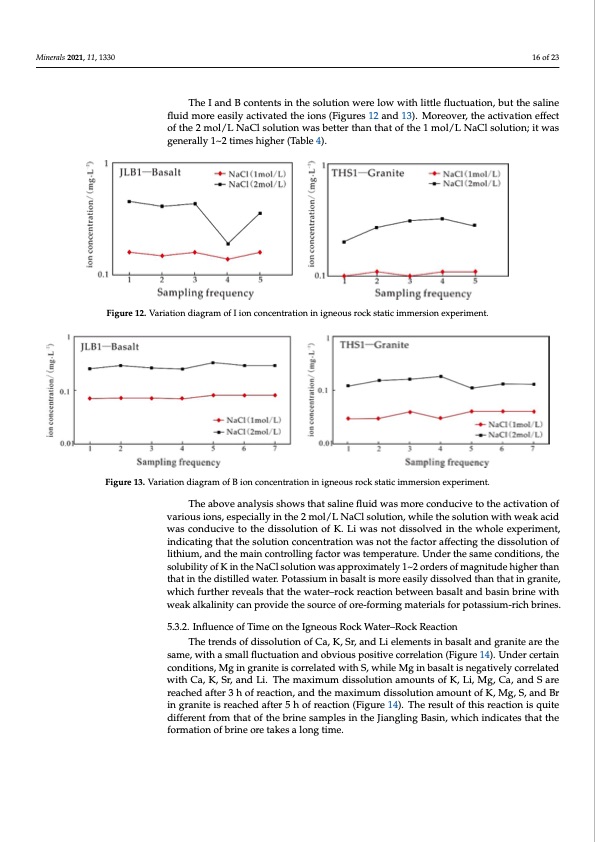
Minerals 2021, 11, 1330 16 of 23 Figure 11. Variation diagram of Br ion concentration in igneous rock static immersion experiment. The I and B contents in the solution were low with little fluctuation, but the saline The I and B contents in the solution were low with little fluctuation, but the saline fluid more easily activated the ions (Figures 12 and 13). Moreover, the activation effect fluid more easily activated the ions (Figures 12 and 13). Moreover, the activation effect of of the 2 mol/L NaCl solution was better than that of the 1 mol/L NaCl solution; it was the 2 mol/L NaCl solution was better than that of the 1 mol/L NaCl solution; it was gen- generally 1~2 times higher (Table 4). erally 1~2 times higher (Table 4). Minerals 2021, 11, 1330 Figure 12. Variation diagram of I ion concentration in igneous rock static immersion experiment. 17 of 24 Figure 13. Variation diagram of B ion concentration in igneous rock static immersion experiment. Figure 13. Variation diagram of B ion concentration in igneous rock static immersion experiment. Theaboveanalysis showsthatsalliinefflluiidwaassmoorreeccoonndduucicviveetotoththeeacatcitviavtaiotinonofof vvaarrioioussiioonss,,eesspeciiallyinthe2mol/LNaaCllssoolulutitoionn,,whhilieleththeesosolulutitoinonwwithithwweaekakacaicdid waasscconducivetottheddisissoolulutitoionnofoKf.KL.iLwiawsansotndoitssdoilsvseodlviendthinewthheowleheoxlpeereixmpenrtim,ine-nt, inddiciactaintigngthtahtathtehesosolulutitoinoncocnocnecnetnrtartaiotinonwwasasnontothtehefafcatcotrorafaffefcetcintigngthtehedidsissosloultuiotinonofof lithium, and the main controlling factor was temperature. Under the same conditions, the lithium, and the main controlling factor was temperature. Under the same conditions, the solubility of K in the NaCl solution was approximately 1~2 orders of magnitude higher solubility of K in the NaCl solution was approximately 1~2 orders of magnitude higher than than that in the distilled water. Potassium in basalt is more easily dissolved than that in that in the distilled water. Potassium in basalt is more easily dissolved than that in granite, granite, which further reveals that the water–rock reaction between basalt and basin which further reveals that the water–rock reaction between basalt and basin brine with brine with weak alkalinity can provide the source of ore-forming materials for potassi- weak alkalinity can provide the source of ore-forming materials for potassium-rich brines. um-rich brines. 5.3.2. Influence of Time on the Igneous Rock Water–Rock Reaction formation of brine ore takes a long time. 5.3.2. Influence of Time on the Igneous Rock Water–Rock Reaction The trends of dissolution of Ca, K, Sr, and Li elements in basalt and granite are the same,TwheithtreansdmsaollfflduiscstoulautionaonfdCoa,bKvi,oSurs, apnodsitLivielceomrreenltastionnb(aFsiaglutraen1d4g).raUnnitdeearrceetrhtaein csoanmdeit,iwonitsh,MasgminallgfrlauncittueaitsiocnorarnedlaotebdviwouitshpSo,switihvielecoMrrgeliantiboans(aFltigiusrnee1g4a)t.iUvenldyecrocrerretlaitned wcoitnhdCitaio,nKs,MSrg,aindgrLain.itTehisecmorarxeilmateudmwditihssSo,luwthioilneaMmgoiunnbtassoalftKis,nLeig,aMtigv,elCyac,oarnredlaSteadre rweaictheCdaa,fKte,rS3r,hanodfrLeia.cTtihoen,maanxdimthuemmdaixssimolutmiondiasmsoolutnitosnoafmKo,uLni,tMofgK,C,Ma,ga,nSd,aSnadreBr inregacrhanediteafitsere3ahchoefdreafctteiron5,hanodftrheeacmtiaoxnim(Fuimgudreis1so4l)u.tTiohnearmesouulnttooffthKi,sMrega,cSt,ioandisBqruinite dgirffaenrietentisfroemachthedataoftfetrh5ehbroifnreascatmiopnle(Fsiginurtehe14J)ia.nTghleinregsuBlatsoinf,thwishricehacitniodniciastqesuitheadtitfh-e foferrmenatiofrnomof bthrainteoofrtehteakbersinaelosanmgptilmese.in the Jiangling Basin, which indicates that thePDF Image | Origin of Lithium Potassium Rich Brines in the Jianghan
PDF Search Title:
Origin of Lithium Potassium Rich Brines in the JianghanOriginal File Name Searched:
minerals-11-01330-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |