
PDF Publication Title:
Text from PDF Page: 014
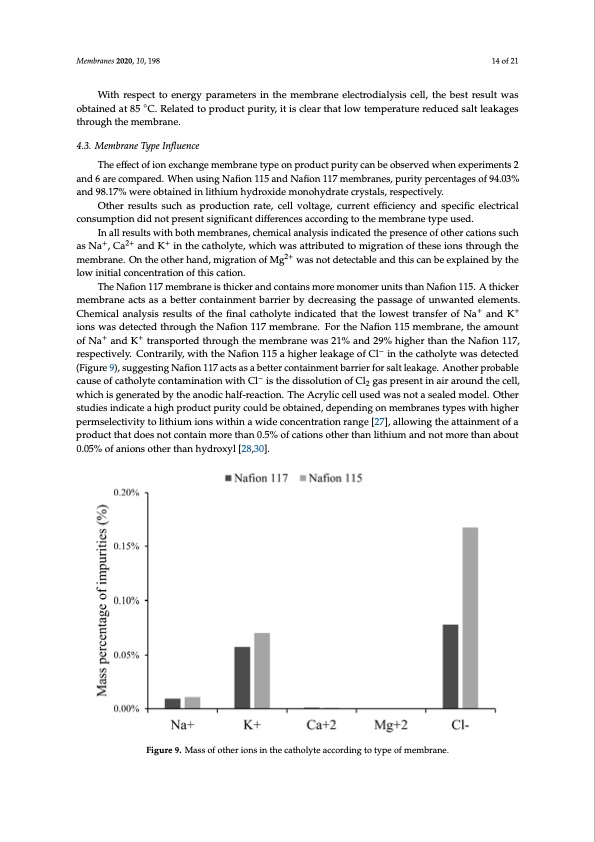
Membranes 2020, 10, 198 14 of 21 With respect to energy parameters in the membrane electrodialysis cell, the best result was obtained at 85 ◦C. Related to product purity, it is clear that low temperature reduced salt leakages through the membrane. 4.3. Membrane Type Influence The effect of ion exchange membrane type on product purity can be observed when experiments 2 and 6 are compared. When using Nafion 115 and Nafion 117 membranes, purity percentages of 94.03% and 98.17% were obtained in lithium hydroxide monohydrate crystals, respectively. Other results such as production rate, cell voltage, current efficiency and specific electrical consumption did not present significant differences according to the membrane type used. In all results with both membranes, chemical analysis indicated the presence of other cations such as Na+, Ca2+ and K+ in the catholyte, which was attributed to migration of these ions through the membrane. On the other hand, migration of Mg2+ was not detectable and this can be explained by the low initial concentration of this cation. The Nafion 117 membrane is thicker and contains more monomer units than Nafion 115. A thicker membrane acts as a better containment barrier by decreasing the passage of unwanted elements. Chemical analysis results of the final catholyte indicated that the lowest transfer of Na+ and K+ ions was detected through the Nafion 117 membrane. For the Nafion 115 membrane, the amount of Na+ and K+ transported through the membrane was 21% and 29% higher than the Nafion 117, respectively. Contrarily, with the Nafion 115 a higher leakage of Cl− in the catholyte was detected (Figure 9), suggesting Nafion 117 acts as a better containment barrier for salt leakage. Another probable cause of catholyte contamination with Cl− is the dissolution of Cl2 gas present in air around the cell, which is generated by the anodic half-reaction. The Acrylic cell used was not a sealed model. Other studies indicate a high product purity could be obtained, depending on membranes types with higher permselectivity to lithium ions within a wide concentration range [27], allowing the attainment of a product that does not contain more than 0.5% of cations other than lithium and not more than about Membranes 2020, 10, x FOR PEER REVIEW 15 of 22 0.05% of anions other than hydroxyl [28,30]. Figure 9. Mass of other ions in the catholyte according to type of membrane. Figure 9. Mass of other ions in the catholyte according to type of membrane. 4.4. Cathode Material Influence The effect of the cathode material on energetic parameters (cell voltage, current efficiency and specific electrical consumption) can be observed by comparing experiments 4 and 5 (see Table 3). Use of nickel as a cathode has some advantages. It was observed that the current efficiency was 5% higher, the obtained production rate of LiOH was 23% higher, and the specific electricalPDF Image | Battery Grade Li Hydroxide by Membrane Electrodialysis
PDF Search Title:
Battery Grade Li Hydroxide by Membrane ElectrodialysisOriginal File Name Searched:
membranes-10-00198.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |