
PDF Publication Title:
Text from PDF Page: 015
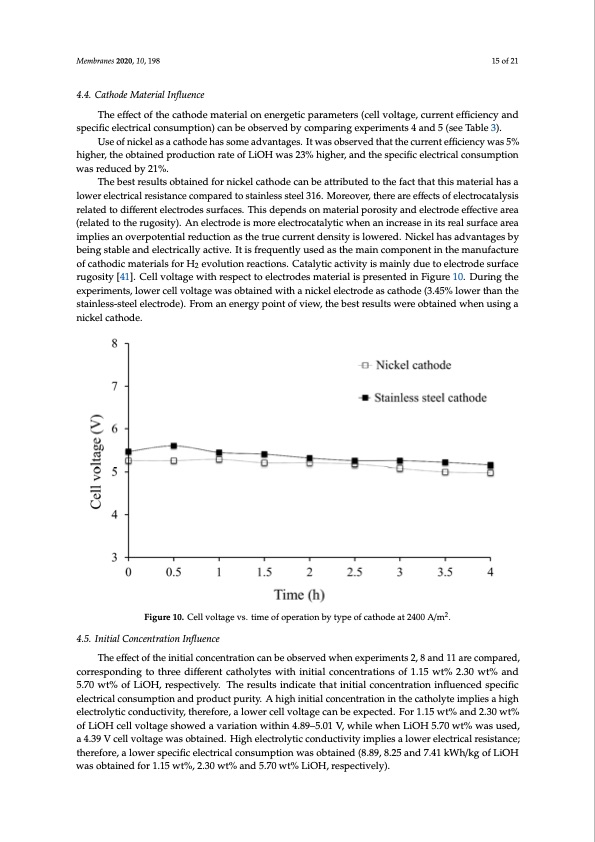
Membranes 2020, 10, 198 15 of 21 4.4. Cathode Material Influence The effect of the cathode material on energetic parameters (cell voltage, current efficiency and specific electrical consumption) can be observed by comparing experiments 4 and 5 (see Table 3). Use of nickel as a cathode has some advantages. It was observed that the current efficiency was 5% higher, the obtained production rate of LiOH was 23% higher, and the specific electrical consumption was reduced by 21%. The best results obtained for nickel cathode can be attributed to the fact that this material has a lower electrical resistance compared to stainless steel 316. Moreover, there are effects of electrocatalysis related to different electrodes surfaces. This depends on material porosity and electrode effective area (related to the rugosity). An electrode is more electrocatalytic when an increase in its real surface area implies an overpotential reduction as the true current density is lowered. Nickel has advantages by being stable and electrically active. It is frequently used as the main component in the manufacture of cathodic materials for H2 evolution reactions. Catalytic activity is mainly due to electrode surface rugosity [41]. Cell voltage with respect to electrodes material is presented in Figure 10. During the experiments, lower cell voltage was obtained with a nickel electrode as cathode (3.45% lower than the Msteaminbrlaenses-2s0t2e0e,l1e0,lexcFtOroRdPeE)E.RFRroEVmIEaWn energy point of view, the best results were obtained when u1s6inofg2a2 nickel cathode. Figure 10. Cell voltage vs. time of operation by type of cathode at 2400 A/m2. Figure 10. Cell voltage vs time of operation by type of cathode at 2400 A/m2. 4.5. Initial Concentration Influence 4.5. Initial Concentration Influence The effect of the initial concentration can be observed when experiments 2, 8 and 11 are compared, correTshpeonedffiencgt tof tthhreeindiitffiaelrecnotncaenthtroalytitoens wcainthbieniotibasl ecrovnecdenwtrhaetnionesxpoefri1m.1e5nwtst%2, 28.3a0nwd t%11 aanrde c5o.7m0 pwart%ed,ocf oLrirOesHp,onredsipnegctiovethlyr.eeTdheiffrerseunltscainthdoiclyatesthwaithiniintitailalcocnocnecnentrtartaitoionnisnflofue1n.1c5edwst%pec2i.fi30c welet%ctraicnadl c5o.7n0suwmtp%tionf LainOdHp,roredsupcetcptiuvreilty. AThheigrhesiunlitsiailncdoinccaetenttrhaatitoinitniatlhceocnacthenotlyratetioimnpinlifelsuaenhciegdh seplectirfoiclyetilcectorincdaluctoivnistuym,thpetiroenfoaren,daplorwodeurctelpluvroiltya.geAcahnigbheienxitpieacltceodn.cFeonrtr1a.1ti5onwti%n tahnedc2a.3th0owlytt%e iomf pLliiOesHacheilglhvoeletacgtreolsyhtoicwceodndauvcatriviaityio,nthweriethfoinre4, .a89lo–w5.e0r1cVe,llwvhoillteagwehcean LbeiOeHxp5e.c7t0edw. tF%orw1.a1s5uwset%d, an4d.329.3V0cweltl%volftLagiOeHwacselolbvtoalitnaegde.sHhoigwheedleacvtraorliyaticoncownidtuhicntiv4.i8ty9–im5.0p1liVes, wa lhoiwleewr helenctLricOaHl re5s.7is0tawntc%e; wthaesreufsoerde, a l4o.3w9eVr scpeellcvifiocltealgeectwriacsalocbotnaisnuemd.pHtioignhwelaescotrbotlayitnicedco(n8d.8u9c, t8iv.2i5tyainmdp7l.i4e1s akWlowh/ekrgeolefcLtriOicaHl rweasissotabntcaein; tehdefroerfo1r.e1,5awlotw%e, r2.s3p0ewcifti%c ealnecdtr5i.c7a0l wcotn%suLmiOpHtio, rnewspaescotibvtealiyn)e. d (8.89, 8.25 and 7.41 kWh/kg of LiOH was obtained for 1.15 wt%, 2.30 wt% and 5.70 wt% LiOH, respectively). On the other hand, as can be observed in Figure 11, a high initial concentration in the catholyte adversely affects purity of the LiOH solution and therefore less product purity is obtained. This could be attributed to the fact that, for a high LiOH concentration of 5.7 wt%, counterion condensation on the membrane can occur, decreasing its permselectivity [26]. This could also be related to a greaterPDF Image | Battery Grade Li Hydroxide by Membrane Electrodialysis
PDF Search Title:
Battery Grade Li Hydroxide by Membrane ElectrodialysisOriginal File Name Searched:
membranes-10-00198.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |