
PDF Publication Title:
Text from PDF Page: 013
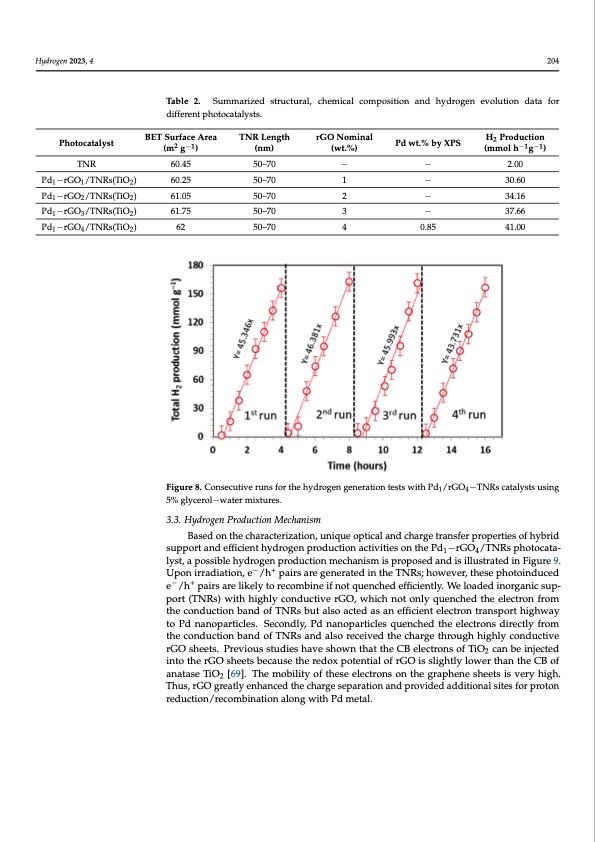
Hydrogen 2023, 4 Photocatalyst time period of 16 h without any sign of deactivation, as shown in Figure 8. A considerable increase in rate was observed for the second and third runs and a rate of 46 mmol g−1h−1 was achieved. This indicates a very high stability and activity of our best performing 204 photocatalyst with time. Ongoing from the third to fourth run, a slight decrease in line slope was observed due to the deposition of photocatalyst particles (clearly seen with the naked eye) on the reactor walls with time, thus scattering away a small fraction of in- Table 2. Summarized structural, chemical composition and hydrogen evolution data for coming light radiation, and also potentially due to the decrease in the sacrificial reagent. different photocatalysts. Table 2. Summarized structural, chemical composition and hydrogen evolution data for different 1 1TNR2 60.25 50–70 − 1 2 3 4 1 2− − 30.60 30.60 34.16 Pd P−dr1G−OrG/OT1/NTRNsR(Tsi(OTiO) 2) 122 61.0650.25 Pd1−rGO2/TNRs(TiO2) 61.05 50–70 − − 0.85 34.16 37.66 41.00 Pd1−rGO3/TNRs(TiO2) Pd1−rGO3/TNRs(TiO2) 61.75 50–70 3 4 photocatalysts. (m2 g−1) BET Surface TNRLength 2 Pdwt.%byXPS H Production BETSurfaceArea rGONominal (wt.%) 60.45 50–70 50–70 2.00 (nm) Pd wt.% by (mmol h−1g−1) H2 Production PThNotRocatalyst Pd −rGO /TNRs(TiO ) TNR Length rGO Nominal 60.45 50–70 − − 2.00 Area (m2 g−1) (nm) (wt.%) XPS − (mmol h−1g−1) − − 37.66 Pd1−rGO4/TNRs(TiO2) Pd1−rGO4/TNRs(TiO2) 61.75 50–70 0.85 41.00 62 62 50–70 50–70 Figure8..ConsseeccuutitviveerurunnssfoforrththeehyhdyrdorgoegnengegnenraertiaotniotnesttesswtsiwthitPhdP1/drG/Or4G−TONR−sTcNatRaslycsatstaulysisntgs using 5% glycerol−water mixtures. 14 5% glycerol−water mixtures. 3.3. HydrrooggeennPProrodduuctcitoinonMMecehcahnainsmism Basedontthecharraacctteerrizizaatitoionn,,uunniqiquueeoopptitciaclaalnadndchcahragregetrtarnasnfsefreprrporpoepretiretsieosfohfyh-ybrid sburipdpsourptpaonrdtaenffidceieffnictihenytdhryodgreongpenropdruocdtuioctnioanctaicvtitvieitsieosnonthtehePdPd−1−rGO4/TTNNRRsspphohtot-ocata- catalyst, a possible hydrogen production mechanism is proposed and is illustrated in lyst, a possible hydrogen production mechanism is proposed and is illustrated in Figure 9. −+−+ Figure 9. Upon irradiation, e /h pairs are generated in the TNRs; however, these pho- Upon irradiation, e /h pairs are generated in the TNRs; however, these photoinduced to−indu+ced e−/h+ pairs are likely to recombine if not quenched efficiently. We loaded in- e /h pairs are likely to recombine if not quenched efficiently. We loaded inorganic sup- organic support (TNRs) with highly conductive rGO, which not only quenched the elec- port (TNRs) with highly conductive rGO, which not only quenched the electron from tron from the conduction band of TNRs but also acted as an efficient electron transport the conduction band of TNRs but also acted as an efficient electron transport highway highway to Pd nanoparticles. Secondly, Pd nanoparticles quenched the electrons directly to Pd nanoparticles. Secondly, Pd nanoparticles quenched the electrons directly from from the conduction band of TNRs and also received the charge through highly conduc- the conduction band of TNRs and also received the charge through highly conductive tive rGO sheets. Previous studies have shown that the CB electrons of TiO2 can be in- rGO sheets. Previous studies have shown that the CB electrons of TiO2 can be injected jected into the rGO sheets because the redox potential of rGO is slightly lower than the into the rGO sheets because the redox potential of rGO is slightly lower than the CB of CB of anatase TiO2 [69]. The mobility of these electrons on the graphene sheets is very high. anatase TiO2 [69]. The mobility of these electrons on the graphene sheets is very high. Thus, rGO greatly enhanced the charge separation and provided additional sites for proton reduction/recombination along with Pd metal. 14PDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |