
PDF Publication Title:
Text from PDF Page: 014
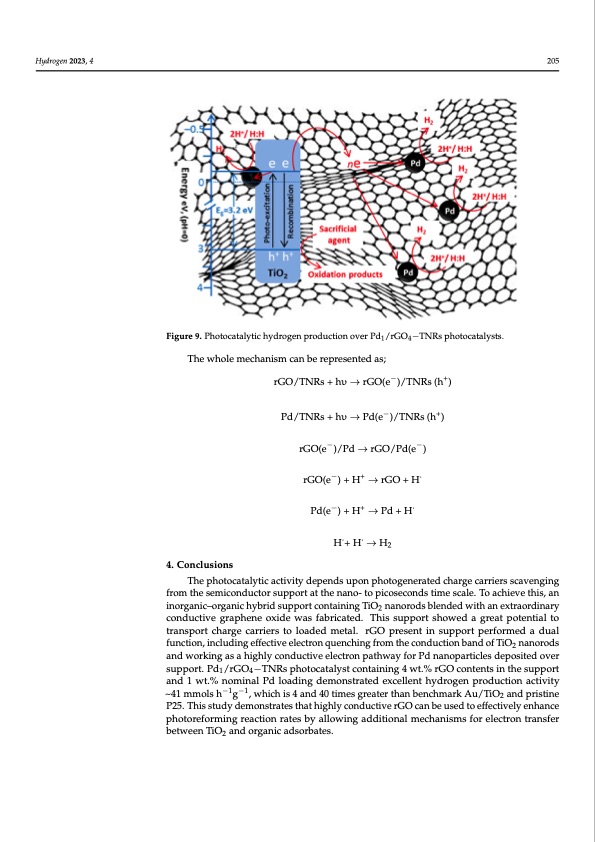
Hydrogen 2023, 4, FOR PEER REVIEW 14 Hydrogen 2023, 4 Thus, rGO greatly enhanced the charge separation and provided additional sites for 205 proton reduction/recombination along with Pd metal. Figure 9. Photocatalytic hydrogen production over Pd1/rGO4−TNRs photocatalysts. Figure 9. Photocatalytic hydrogen production over Pd1/rGO4−TNRs photocatalysts. The whole mechanism can be represented as; The whole mechanism can be represented as; 4. Conclusions rGO/TNRs + hυ → rGO(e−)/TNRs (h+) rGO/TNRs + hυ → rGO(e−)/TNRs (h+) Pd/TNRs + hυ → Pd(e−)/TNRs (h+) rGO(e−)/Pd → rGO/Pd(e−) Pd/TNRs + hυ → Pd(e−)/TNRs (h+) rGO(e−) + H+ → rGO + H ̇ −−+− Pd(e)+H →Pd+H ̇ rGO(e )/Pd→rGO/Pd(e ) H ̇+ H ̇ → H2 rGO(e−) + H+ → rGO + H ̇ The photocatalytic activity depends upon photogenerated charge carriers scaveng- Pd(e−)+H+ →Pd+H ̇ ing from the semiconductor support at the nano- to picoseconds time scale. To achieve this, an inorganic–organic hybrid support containing TiO2 nanorods blended with an H ̇+ H ̇ → H extraordinary conductive graphene oxide was fabri2cated. This support showed a great potential to transport charge carriers to loaded metal. rGO present in support performed 4. Conclusions a dual function, including effective electron quenching from the conduction band of TiO2 nanorods and working as a highly conductive electron pathway for Pd nanoparti- The photocatalytic activity depends upon photogenerated charge carriers scavenging frcolems dthepeosseimtediconvderucstuoprpsourpt.pPodrt1/arGt tOh4e−TnNanRos-ptohoptioccoasteaclyosntdcsotnitmaienisncgal4e.wTto.%acrhGieOvecotnh-is, an inteonrtgsainict–hoergsuapnpicohrtyabnrid1suwptp.%ortncoomnitnaainliPndglToiaOdingandoermodonssbtrleantededexwceitllhenatnheyxdtraoogredninary 2 −1 −1 cpornoductivoneagcrtaivpihtyen~e41oxmidmeolwsahsgfab,rwichaitcehdi.sT4hainsdsu40pptiomretsshgroewatedrtahagnrebaetnpchomteanrtkialto trAaun/sTpiOor2tacnhdaprgriesticnaerrPie2r5s. Ttohislosatdudedy dmeemtaoln.strGatOes pthraetsehnigthilny scuonpdpuocrtivpeerGfoOrmceadn baedual used to effectively enhance photoreforming reaction rates by allowing additional mech- function, including effective electron quenching from the conduction band of TiO2 nanorods anisms for electron transfer between TiO2 and organic adsorbates. and working as a highly conductive electron pathway for Pd nanoparticles deposited over support. Pd1/rGO4−TNRs photocatalyst containing 4 wt.% rGO contents in the support Author Contributions: Conceptualization, I.M., A.I., A.R. and M.A.N. (Muhammad Amtiaz and 1 wt.% nominal Pd loading demonstrated excellent hydrogen production activity Nadeem); Methodology, I.M.; Validation, H.A.; Formal analysis, A.A.; Investigation, I.M., H.U. and ~41mmolsh−1g−1,whichis4and40timesgreaterthanbenchmarkAu/TiO andpristine 2 A.M.; Resources, M.A.N. (Muhammad Arif Nadeem) and M.A.N. (Muhammad Amtiaz Nadeem); P25. This study demonstrates that highly conductive rGO can be used to effectively enhance photoreforming reaction rates by allowing additional mechanisms for electron transfer between TiO2 and organic adsorbates.PDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |