
PDF Publication Title:
Text from PDF Page: 012
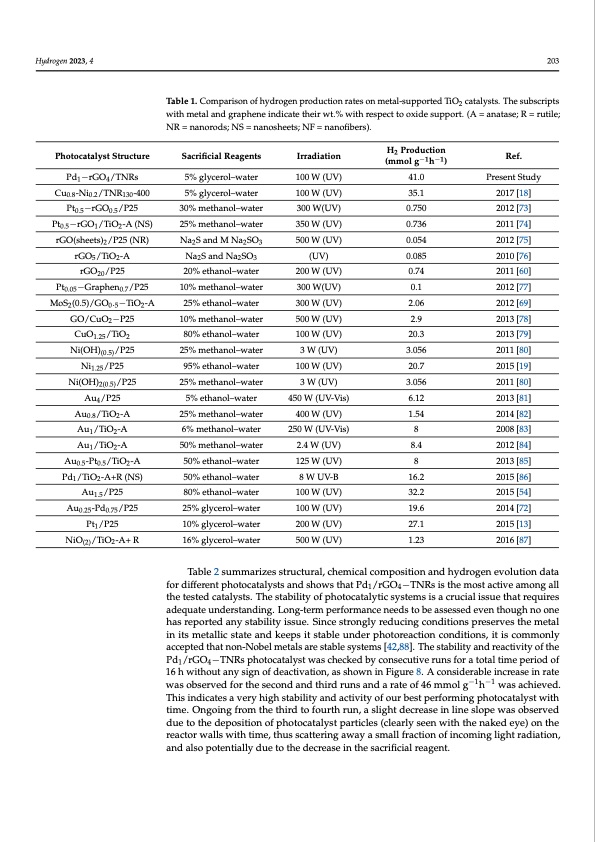
Hydrogen 2023, 4 203 Table 1. Comparison of hydrogen production rates on metal-supported TiO2 catalysts. The subscripts with metal and graphene indicate their wt.% with respect to oxide support. (A = anatase; R = rutile; NR = nanorods; NS = nanosheets; NF = nanofibers). Photocatalyst Structure Pd1 −rGO4 /TNRs Cu0.8 -Ni0.2 /TNR130 -400 Pt0.5 −rGO0.5 /P25 Pt0.5−rGO1/TiO2-A (NS) rGO(sheets)2/P25 (NR) rGO5 /TiO2 -A rGO20 /P25 Pt0.05 −Graphen0.7 /P25 MoS2(0.5)/GO0.5−TiO2-A GO/CuO2 −P25 CuO1.25 /TiO2 Ni(OH)(0.5) /P25 Ni1.25 /P25 Ni(OH)2(0.5) /P25 Au4 /P25 Au0.8 /TiO2 -A Au1 /TiO2 -A Au1 /TiO2 -A Au0.5 -Pt0.5 /TiO2 -A Pd1/TiO2-A+R (NS) Au1.5 /P25 Au0.25 -Pd0.75 /P25 Pt1 /P25 NiO(2)/TiO2-A+ R Sacrificial Reagents 5% glycerol–water 5% glycerol–water 30% methanol–water 25% methanol–water Na2S and M Na2SO3 Na2S and Na2SO3 20% ethanol–water 10% methanol–water 25% ethanol–water 10% methanol–water 80% ethanol–water 25% methanol–water 95% ethanol–water 25% methanol–water 5% ethanol–water 25% methanol–water 6% methanol–water 50% methanol–water 50% ethanol–water 50% ethanol–water 80% ethanol–water 25% glycerol–water 10% glycerol–water 16% glycerol–water Irradiation 100 W (UV) 100 W (UV) 300 W(UV) 350 W (UV) 500 W (UV) (UV) 200 W (UV) 300 W(UV) 300 W (UV) 500 W (UV) 100 W (UV) 3 W (UV) 100 W (UV) 3 W (UV) 450 W (UV-Vis) 400 W (UV) 250 W (UV-Vis) 2.4 W (UV) 125 W (UV) 8 W UV-B 100 W (UV) 100 W (UV) 200 W (UV) 500 W (UV) H2 Production (mmol g−1h−1) 41.0 35.1 0.750 0.736 0.054 0.085 0.74 0.1 2.06 2.9 20.3 3.056 20.7 3.056 6.12 1.54 8 8.4 8 16.2 32.2 19.6 27.1 1.23 Ref. Present Study 2017 [18] 2012 [73] 2011 [74] 2012 [75] 2010 [76] 2011 [60] 2012 [77] 2012 [69] 2013 [78] 2013 [79] 2011 [80] 2015 [19] 2011 [80] 2013 [81] 2014 [82] 2008 [83] 2012 [84] 2013 [85] 2015 [86] 2015 [54] 2014 [72] 2015 [13] 2016 [87] Table 2 summarizes structural, chemical composition and hydrogen evolution data for different photocatalysts and shows that Pd1/rGO4−TNRs is the most active among all the tested catalysts. The stability of photocatalytic systems is a crucial issue that requires adequate understanding. Long-term performance needs to be assessed even though no one has reported any stability issue. Since strongly reducing conditions preserves the metal in its metallic state and keeps it stable under photoreaction conditions, it is commonly accepted that non-Nobel metals are stable systems [42,88]. The stability and reactivity of the Pd1/rGO4−TNRs photocatalyst was checked by consecutive runs for a total time period of 16 h without any sign of deactivation, as shown in Figure 8. A considerable increase in rate was observed for the second and third runs and a rate of 46 mmol g−1h−1 was achieved. This indicates a very high stability and activity of our best performing photocatalyst with time. Ongoing from the third to fourth run, a slight decrease in line slope was observed due to the deposition of photocatalyst particles (clearly seen with the naked eye) on the reactor walls with time, thus scattering away a small fraction of incoming light radiation, and also potentially due to the decrease in the sacrificial reagent.PDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |