
PDF Publication Title:
Text from PDF Page: 011
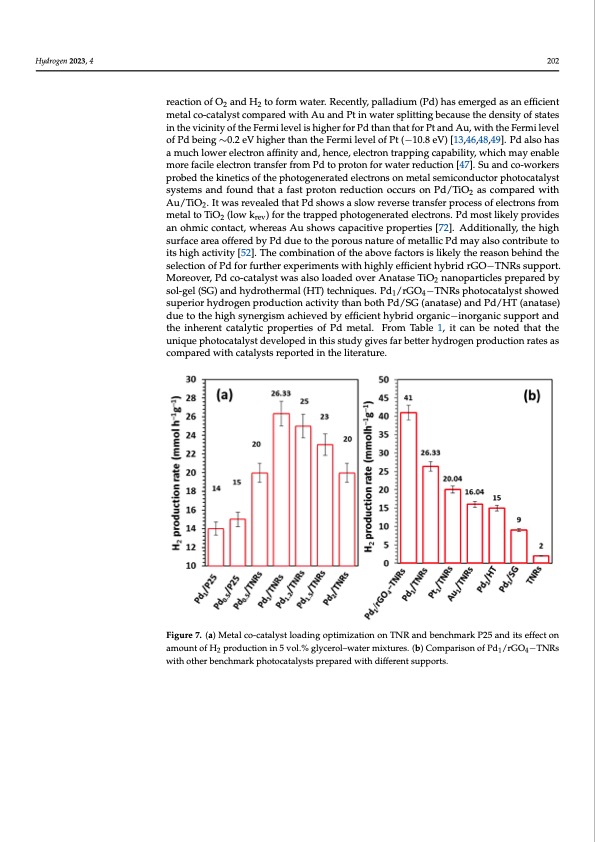
Hydrogen 2023, 4 202 reaction of O2 and H2 to form water. Recently, palladium (Pd) has emerged as an efficient metal co-catalyst compared with Au and Pt in water splitting because the density of states in the vicinity of the Fermi level is higher for Pd than that for Pt and Au, with the Fermi level of Pd being ∼0.2 eV higher than the Fermi level of Pt (−10.8 eV) [13,46,48,49]. Pd also has a much lower electron affinity and, hence, electron trapping capability, which may enable more facile electron transfer from Pd to proton for water reduction [47]. Su and co-workers probed the kinetics of the photogenerated electrons on metal semiconductor photocatalyst systems and found that a fast proton reduction occurs on Pd/TiO as compared with Hydrogen 2023, 4, FOR PEER REVIEW 2 11 Au/TiO2. It was revealed that Pd shows a slow reverse transfer process of electrons from metal to TiO2 (low krev) for the trapped photogenerated electrons. Pd most likely provides an ohmic contact, whereas Au shows capacitive properties [72]. Additionally, the high over TNRs was observed, and after that there was a gradual decrease in activity up to 2 surface area offered by Pd due to the porous nature of metallic Pd may also contribute to wt.% loading. This decrease in activity with an increased amount of metal loading has its high activity [52]. The combination of the above factors is likely the reason behind the also been observed by others [18]. It has been suggested that metal particle size, increased selection of Pd for further experiments with highly efficient hybrid rGO−TNRs support. surface defect concentration and shadowing of incoming light by metal nanoparticles Moreover, Pd co-catalyst was also loaded over Anatase TiO2 nanoparticles prepared by might be behind this. To clarify this, a systematic study using slurry phase reactions as sol-gel (SG) and hydrothermal (HT) techniques. Pd1/rGO4−TNRs photocatalyst showed swueplleraisormhoyderlogsuenrfapcreosdiuscrtieoqnuaircetdiv.iAtytlohwanerboatmhoPudn/tSoGf(hayndartoagse)napnrdoPdduc/tHioTn(wanaastaslseo) dobuseetrovetdheath0ig.5hwsyt.n%erPgdismloaadcihnige.vTehdisbyisedfufieciteontheyabvriadilaobrgilaitnyico−f fienworegraancitcivseupmpeotartl sainteds tohverinThNeRresnftorcaptraolytotinc rpedroupcetirotniesorohf yPddromgentarle. coFmrobminaTtaiobnle. A1,s iat coansebqeunenocted, atshuaittatbhle uconniqteunetpohfoPtodcaistaclyrustcdiaelvfeolropthede ionpthimisiszteuddyphgoivtoecsaftaarlybteitcteprehryfodrmogaenceprofdPudct/iroGnOra−tTeNs aRs cpohmotpoacraetdalwysitsh. catalysts reported in the literature. Figure 7. (a) Metal co-catalyst loading optimization on TNR and benchmark P25 and its effect on Figure 7. (a) Metal co-catalyst loading optimization on TNR and benchmark P25 and its effect on amount of H production in 5 vol.% glycerol–water mixtures. (b) Comparison of Pd /rGO −TNRs 214 amount of H2 production in 5 vol.% glycerol–water mixtures. (b) Comparison of Pd1/rGO4−TNRs with other benchmark photocatalysts prepared with different supports. with other benchmark photocatalysts prepared with different supports. Figure 7b shows the comparison between three benchmark co-catalyst (Pt, Pd, Au) loaded TNRs and Pd1/rGO4−TNRs photocatalysts in the current study. In 5 wt.% glycer- ol–water system, 1 wt.% Pd/TNRs demonstrated superior hydrogen production than the other two[13]. Under present conditions, the order of activity was observed as Pd ˃ Pt ˃ Au. It has been established that metal co-catalyst particle size has no considerable effect on the photo-catalytic activity , so the key factor to consider might be the work function of Pd, Pt, Au and TNRs (5.7, 5.2, 5.1 and 4.26) [50]. Theoretically, Pt should be more ac- tive when compared with Pd and Au due to the larger height of the Schottky barrier and other intrinsic catalytic properties. The main reason behind this might be the favorable thermal back reaction of O2 and H2 to form water. Recently, palladium (Pd) has emerged as an efficient metal co-catalyst compared with Au and Pt in water splitting because the density of states in the vicinity of the Fermi level is higher for Pd than that for Pt and Au, with the Fermi level of Pd being ∼0.2 eV higher than the Fermi level of Pt (−10.8 eV)PDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |