
PDF Publication Title:
Text from PDF Page: 010
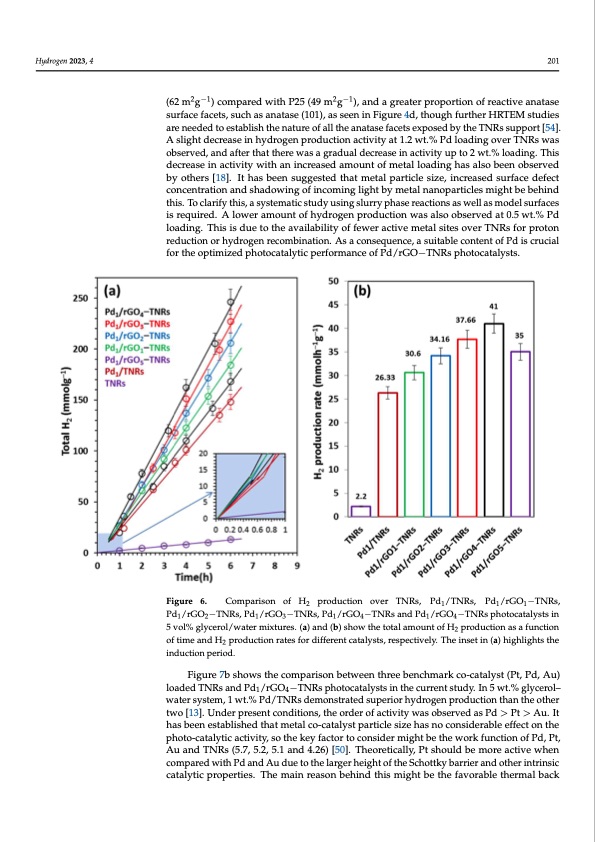
ydrogen 2H0y2d3r,og4e,nF2O02R3,P4EER REVIEW 201 (62 m2g−1) compared with P25 (49 m2g−1), and a greater proportion of reactive anatase erated charge carriers. After the introduction of 1 wt.% of Pd, the H2 production ra surface facets, such as anatase (101), as seen in Figure 4d, though further HRTEM studies rapidly increased to 26.33 mmol h−1g−1 as the Pd metal suppressed the charge carrier are needed to establish the nature of all the anatase facets exposed by the TNRs support [54]. recoAmsblignhatidoencrebaysesicnahvyednrgoignegn pthroedcuocntiodnuaccttioivnitybatn1d.2ewlet.c%trPodnsloadnidngaocvtedr TaNsRas cwaatshodic r duction/recombination site for protons. The addition of layered rGO with TNRs suppo observed, and after that there was a gradual decrease in activity up to 2 wt.% loading. This resulted in a significant improvement of hydrogen production activity, and the content decrease in activity with an increased amount of metal loading has also been observed by others [18]. It has been suggested that metal particle size, increased surface defect rGO in TNRs had a significant influence in this unique hybrid combination. In the pre concentration and shadowing of incoming light by metal nanoparticles might be behind ence of rGO (1.0 wt.%) in the hybrid support, the activity was enhanced to 30.66 mm this. To clarify this, a systematic study using slurry phase reactions as well as model surfaces −1 −1 h gis.rTeqhueirheyd.dArolgoewnerparmodounctiofnhyradtreoggernapdruoadlulyctiioncrweassealdsowoibtshertvheediantc0r.e5awste.%inPdrGO co −1 −1 tentslo,adnidngt.hTehhisigishdeusettvoatlhueeawvaailsabaiclihtyieovfefdewaetr4acwtivt.e%m(e4ta1lmsitmesoolvherTgN)R;saffotrerprtohtaotn,thera started to decrease due to the shadowing effect on support (discussed later). reduction or hydrogen recombination. As a consequence, a suitable content of Pd is crucial for the optimized photocatalytic performance of Pd/rGO−TNRs photocatalysts. Figure 6. Comparison of H production over TNRs, Pd /TNRs, Pd /rGO −TNRs, 2111 Figure 6. Comparison of H2 production over TNRs, Pd1/TNRs, Pd1/rGO1−TNRs, Pd1/rGO2−TNR Pd /rGO −TNRs, Pd /rGO −TNRs, Pd /rGO −TNRs and Pd /rGO −TNRs photocatalysts in Pd1/rGO3−TNRs, Pd1/rGO4−TNRs and Pd1/rGO4−TNRs photocatalysts in 5 vol% glycerol/wat 12131414 5 vol% glycerol/water mixtures. (a) and (b) show the total amount of H mixtures. Figures (a) and (b) show the total amount of H2 production as a function of time and production as a function of time and H2 production rates for different catalysts, respectively. The inset in (a) highlights the production rates for different catalysts, respectively. The inset in (a) highlights the induction per od. inductionperiod. Figure 7b shows the comparison between three benchmark co-catalyst (Pt, Pd, Au) loaded TNRs and Pd /rGO −TNRs photocatalysts in the current study. In 5 wt.% glycerol– Figure 7a explai1ns the4 effect of the overall Pd loading amount over TNRs. T water system, 1 wt.% Pd/TNRs demonstrated superior hydrogen production than the other highest rate of hydrogen production was achieved over TNRs at an overall nomin two [13]. Under present conditions, the order of activity was observed as Pd > Pt > Au. It metal loading amount of 1 wt.%. Recent studies proved that 0.5 wt.% Pd loading over P2 has been established that metal co-catalyst particle size has no considerable effect on the nanoparticles showed the maximum rate of hydrogen production [13]. At the same tim photo-catalytic activity, so the key factor to consider might be the work function of Pd, Pt, bothA1uandT0N.5Rsw(t5..%7,5P.2d,/5P.125ansdho4.w26e)d[5l0e].ssThaecotirvetiitcyaltlyh,aPnts1howutld.%beomnoPred/aTctNivResw.hTehne reaso behind the superior activities of TNRs compared with P25 is ascribed to their excelle compared with Pd and Au due to the larger height of the Schottky barrier and other intrinsic catalytic properties. The main reason− be+hind this might be the favorable thermal back charge transportation and longer e /h lifetime due to following features: 1. effecti vectorial transfer of photogenerated charges due to a nanorod structure; 2. the charg carriers can not only move easily along the longitudinal direction, but they also have t travel short distances to emerge at the surface transversely; 3. lower number of surfa defects, thus a small number of recombination sites; 4. TNRs support possesses hig 2 1 t s e r o s o n t e H h a e n v cPDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |