
PDF Publication Title:
Text from PDF Page: 002
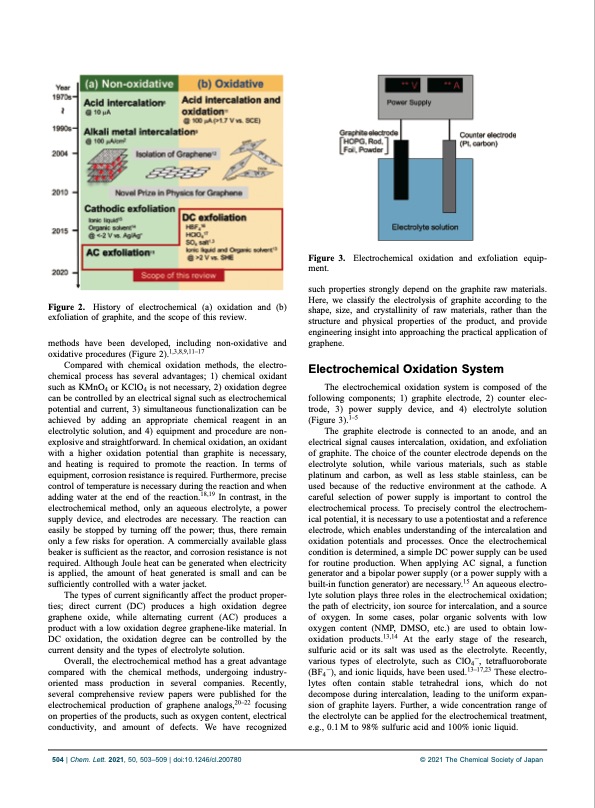
Figure 2. History of electrochemical (a) oxidation and (b) exfoliation of graphite, and the scope of this review. methods have been developed, including non-oxidative and oxidative procedures (Figure 2).1,3,8,9,1117 Compared with chemical oxidation methods, the electro- chemical process has several advantages; 1) chemical oxidant such as KMnO4 or KClO4 is not necessary, 2) oxidation degree can be controlled by an electrical signal such as electrochemical potential and current, 3) simultaneous functionalization can be achieved by adding an appropriate chemical reagent in an electrolytic solution, and 4) equipment and procedure are non- explosive and straightforward. In chemical oxidation, an oxidant with a higher oxidation potential than graphite is necessary, and heating is required to promote the reaction. In terms of equipment, corrosion resistance is required. Furthermore, precise control of temperature is necessary during the reaction and when adding water at the end of the reaction.18,19 In contrast, in the electrochemical method, only an aqueous electrolyte, a power supply device, and electrodes are necessary. The reaction can easily be stopped by turning off the power; thus, there remain only a few risks for operation. A commercially available glass beaker is sufficient as the reactor, and corrosion resistance is not required. Although Joule heat can be generated when electricity is applied, the amount of heat generated is small and can be sufficiently controlled with a water jacket. The types of current significantly affect the product proper- ties; direct current (DC) produces a high oxidation degree graphene oxide, while alternating current (AC) produces a product with a low oxidation degree graphene-like material. In DC oxidation, the oxidation degree can be controlled by the current density and the types of electrolyte solution. Overall, the electrochemical method has a great advantage compared with the chemical methods, undergoing industry- oriented mass production in several companies. Recently, several comprehensive review papers were published for the electrochemical production of graphene analogs,2022 focusing on properties of the products, such as oxygen content, electrical conductivity, and amount of defects. We have recognized Figure 3. Electrochemical oxidation and exfoliation equip- ment. such properties strongly depend on the graphite raw materials. Here, we classify the electrolysis of graphite according to the shape, size, and crystallinity of raw materials, rather than the structure and physical properties of the product, and provide engineering insight into approaching the practical application of graphene. Electrochemical Oxidation System The electrochemical oxidation system is composed of the following components; 1) graphite electrode, 2) counter elec- trode, 3) power supply device, and 4) electrolyte solution (Figure 3).15 The graphite electrode is connected to an anode, and an electrical signal causes intercalation, oxidation, and exfoliation of graphite. The choice of the counter electrode depends on the electrolyte solution, while various materials, such as stable platinum and carbon, as well as less stable stainless, can be used because of the reductive environment at the cathode. A careful selection of power supply is important to control the electrochemical process. To precisely control the electrochem- ical potential, it is necessary to use a potentiostat and a reference electrode, which enables understanding of the intercalation and oxidation potentials and processes. Once the electrochemical condition is determined, a simple DC power supply can be used for routine production. When applying AC signal, a function generator and a bipolar power supply (or a power supply with a built-in function generator) are necessary.15 An aqueous electro- lyte solution plays three roles in the electrochemical oxidation; the path of electricity, ion source for intercalation, and a source of oxygen. In some cases, polar organic solvents with low oxygen content (NMP, DMSO, etc.) are used to obtain low- oxidation products.13,14 At the early stage of the research, sulfuric acid or its salt was used as the electrolyte. Recently, various types of electrolyte, such as ClO41, tetrafluoroborate (BF41), and ionic liquids, have been used.1317,23 These electro- lytes often contain stable tetrahedral ions, which do not decompose during intercalation, leading to the uniform expan- sion of graphite layers. Further, a wide concentration range of the electrolyte can be applied for the electrochemical treatment, e.g., 0.1 M to 98% sulfuric acid and 100% ionic liquid. 504 | Chem. Lett. 2021, 50, 503–509 | doi:10.1246/cl.200780 © 2021 The Chemical Society of JapanPDF Image | Electrochemical Production of Graphene Analogs
PDF Search Title:
Electrochemical Production of Graphene AnalogsOriginal File Name Searched:
cl-200780.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |