
PDF Publication Title:
Text from PDF Page: 172
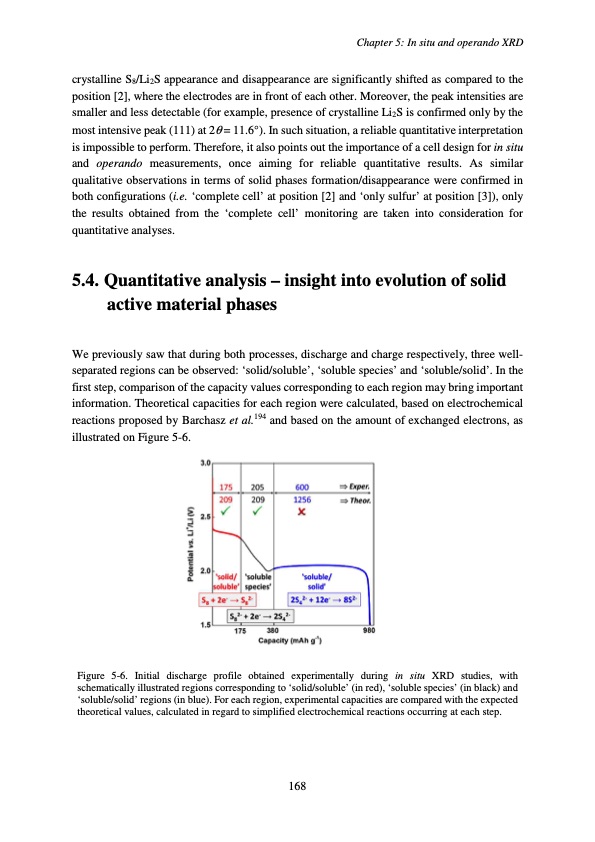
crystalline S8/Li2S appearance and disappearance are significantly shifted as compared to the position [2], where the electrodes are in front of each other. Moreover, the peak intensities are smaller and less detectable (for example, presence of crystalline Li2S is confirmed only by the most intensive peak (111) at 2θ = 11.6°). In such situation, a reliable quantitative interpretation is impossible to perform. Therefore, it also points out the importance of a cell design for in situ and operando measurements, once aiming for reliable quantitative results. As similar qualitative observations in terms of solid phases formation/disappearance were confirmed in both configurations (i.e. ‘complete cell’ at position [2] and ‘only sulfur’ at position [3]), only the results obtained from the ‘complete cell’ monitoring are taken into consideration for quantitative analyses. 5.4. Quantitative analysis – insight into evolution of solid active material phases We previously saw that during both processes, discharge and charge respectively, three well- separated regions can be observed: ‘solid/soluble’, ‘soluble species’ and ‘soluble/solid’. In the first step, comparison of the capacity values corresponding to each region may bring important information. Theoretical capacities for each region were calculated, based on electrochemical reactions proposed by Barchasz et al.194 and based on the amount of exchanged electrons, as illustrated on Figure 5-6. Figure 5-6. Initial discharge profile obtained experimentally during in situ XRD studies, with schematically illustrated regions corresponding to ‘solid/soluble’ (in red), ‘soluble species’ (in black) and ‘soluble/solid’ regions (in blue). For each region, experimental capacities are compared with the expected theoretical values, calculated in regard to simplified electrochemical reactions occurring at each step. Chapter 5: In situ and operando XRD 168PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |