
PDF Publication Title:
Text from PDF Page: 171
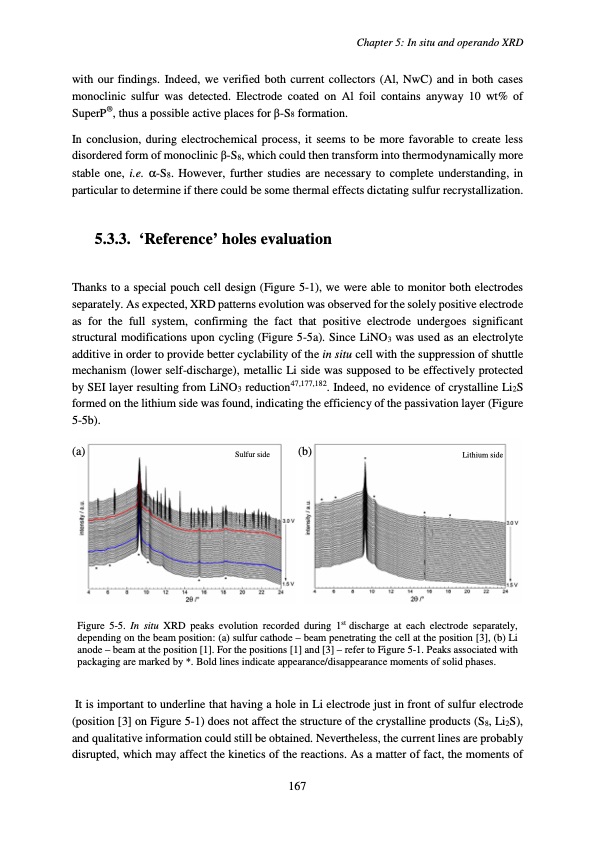
with our findings. Indeed, we verified both current collectors (Al, NwC) and in both cases monoclinic sulfur was detected. Electrode coated on Al foil contains anyway 10 wt% of SuperP®, thus a possible active places for β-S8 formation. In conclusion, during electrochemical process, it seems to be more favorable to create less disordered form of monoclinic β-S8, which could then transform into thermodynamically more stable one, i.e. α-S8. However, further studies are necessary to complete understanding, in particular to determine if there could be some thermal effects dictating sulfur recrystallization. 5.3.3. ‘Reference’ holes evaluation Thanks to a special pouch cell design (Figure 5-1), we were able to monitor both electrodes separately. As expected, XRD patterns evolution was observed for the solely positive electrode as for the full system, confirming the fact that positive electrode undergoes significant structural modifications upon cycling (Figure 5-5a). Since LiNO3 was used as an electrolyte additive in order to provide better cyclability of the in situ cell with the suppression of shuttle mechanism (lower self-discharge), metallic Li side was supposed to be effectively protected by SEI layer resulting from LiNO3 reduction47,177,182. Indeed, no evidence of crystalline Li2S formed on the lithium side was found, indicating the efficiency of the passivation layer (Figure 5-5b). (a) Sulfur side (b) Lithium side Figure 5-5. In situ XRD peaks evolution recorded during 1st discharge at each electrode separately, depending on the beam position: (a) sulfur cathode – beam penetrating the cell at the position [3], (b) Li anode – beam at the position [1]. For the positions [1] and [3] – refer to Figure 5-1. Peaks associated with packaging are marked by *. Bold lines indicate appearance/disappearance moments of solid phases. It is important to underline that having a hole in Li electrode just in front of sulfur electrode (position [3] on Figure 5-1) does not affect the structure of the crystalline products (S8, Li2S), and qualitative information could still be obtained. Nevertheless, the current lines are probably disrupted, which may affect the kinetics of the reactions. As a matter of fact, the moments of Chapter 5: In situ and operando XRD 167PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |