
PDF Publication Title:
Text from PDF Page: 006
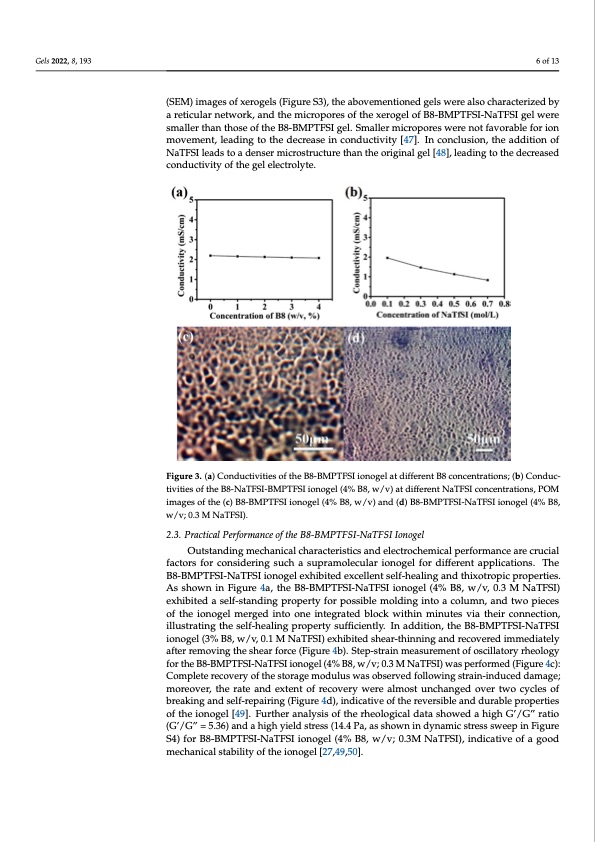
Gels 2022, 8, 193 6 of 13 (SEM) images of xerogels (Figure S3), the abovementioned gels were also characterized by a reticular network, and the micropores of the xerogel of B8-BMPTFSI-NaTFSI gel were smaller than those of the B8-BMPTFSI gel. Smaller micropores were not favorable for ion Gels 2022, 8, x FOR PEER REVIEW movement, leading to the decrease in conductivity [47]. In conclusion, the addition of NaTFSI leads to a denser microstructure than the original gel [48], leading to the decreased conductivity of the gel electrolyte. Figure 3. (a) Conductivities of the B8-BMPTFSI ionogel at different B8 concentrations; (b) Conduc- Figure 3. (a) Conductivities of the B8-BMPTFSI ionogel at different B8 concentrations; (b tivities of the B8-NaTFSI-BMPTFSI ionogel (4% B8, w/v) at different NaTFSI concentrations, POM tivities of the B8-NaTFSI-BMPTFSI ionogel (4% B8, w/v) at different NaTFSI concentrati images of the (c) B8-BMPTFSI ionogel (4% B8, w/v) and (d) B8-BMPTFSI-NaTFSI ionogel (4% B8, images of the (c) B8-BMPTFSI ionogel (4% B8, w/v) and (d) B8-BMPTFSI-NaTFSI ionog w/v; 0.3 M NaTFSI). w/v; 0.3 M NaTFSI). 2.3. Practical Performance of the B8-BMPTFSI-NaTFSI Ionogel 2.3. Practical Performance of the B8-BMPTFSI-NaTFSI Ionogel Outstanding mechanical characteristics and electrochemical performance are crucial factors for considering such a supramolecular ionogel for different applications. The Outstanding mechanical characteristics and electrochemical performance a B8-BMPTFSI-NaTFSI ionogel exhibited excellent self-healing and thixotropic properties. factors for considering such a supramolecular ionogel for different applications As shown in Figure 4a, the B8-BMPTFSI-NaTFSI ionogel (4% B8, w/v, 0.3 M NaTFSI) BMPTFSI-NaTFSI ionogel exhibited excellent self-healing and thixotropic prop exhibited a self-standing property for possible molding into a column, and two pieces osfhtohewinoniongeFligmuerege4dai,ntthoeoBne8-iBntMegPraTteFdSIb-lNocakTwFSitIhinonmoinguetle(s4v%iaBth8e,iwr c/ovn,n0e.c3tiMon,NaTF illustrating the self-healing property sufficiently. In addition, the B8-BMPTFSI-NaTFSI ited a self-standing property for possible molding into a column, and two piec ionogel (3% B8, w/v, 0.1 M NaTFSI) exhibited shear-thinning and recovered immediately ionogel merged into one integrated block within minutes via their connection, ill after removing the shear force (Figure 4b). Step-strain measurement of oscillatory rheology the self-healing property sufficiently. In addition, the B8-BMPTFSI-NaTFSI ion for the B8-BMPTFSI-NaTFSI ionogel (4% B8, w/v; 0.3 M NaTFSI) was performed (Figure 4c): B8, w/v, 0.1 M NaTFSI) exhibited shear-thinning and recovered immediately afte Complete recovery of the storage modulus was observed following strain-induced damage; minogreothvers, htheearaftoeracned(Feixgteunrteo4fbr)e.cSovtepry-swtrearienamlmeoasst urnecmhaenngtedofoovsercitlwlaotocryyclrehs eoof logy f breaking and self-repairing (Figure 4d), indicative of the reversible and durable properties BMPTFSI-NaTFSI ionogel (4% B8, w/v; 0.3 M NaTFSI) was performed (Figure 4 of the ionogel [49]. Further analysis of the rheological data showed a high G’/G” ratio plete recovery of the storage modulus was observed following strain-induced (G’/G” = 5.36) and a high yield stress (14.4 Pa, as shown in dynamic stress sweep in Figure moreover, the rate and extent of recovery were almost unchanged over two S4) for B8-BMPTFSI-NaTFSI ionogel (4% B8, w/v; 0.3M NaTFSI), indicative of a good mbercehaknincagl satnabdilsiteylfo-frtehpeaioirnionggel([F2i7g,4u9r,5e04].d), indicative of the reversible and durable p of the ionogel [49]. Further analysis of the rheological data showed a high G’ (G’/G” = 5.36) and a high yield stress (14.4 Pa, as shown in dynamic stress sweep S4) for B8-BMPTFSI-NaTFSI ionogel (4% B8, w/v; 0.3M NaTFSI), indicative of a chanical stability of the ionogel [27,49,50]. ) o e r . e S u o o c r / i gPDF Image | Thixotropic Ionogel Electrolyte for Sodium Batteries
PDF Search Title:
Thixotropic Ionogel Electrolyte for Sodium BatteriesOriginal File Name Searched:
gels-08-00193-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |