
PDF Publication Title:
Text from PDF Page: 008
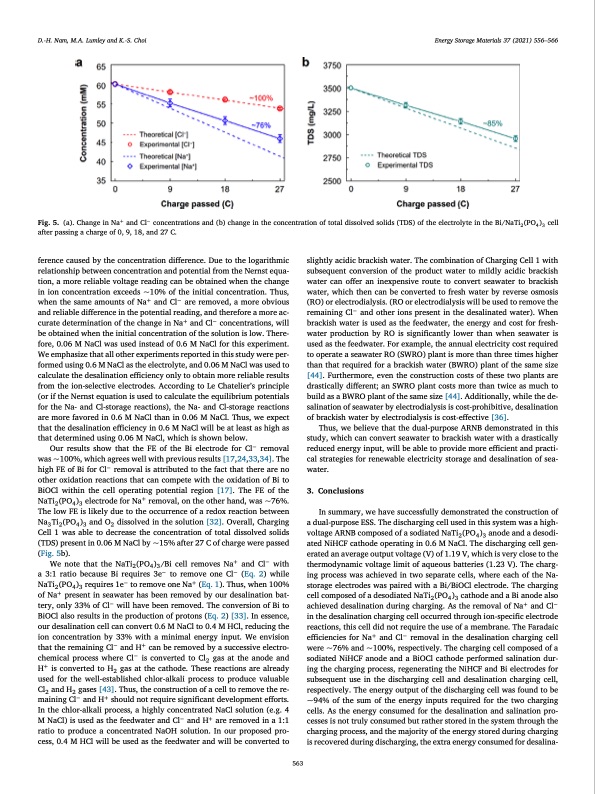
D.-H. Nam, M.A. Lumley and K.-S. Choi Energy Storage Materials 37 (2021) 556–566 Fig. 5. (a). Change in Na+ and Cl− concentrations and (b) change in the concentration of total dissolved solids (TDS) of the electrolyte in the Bi/NaTi2(PO4)3 cell after passing a charge of 0, 9, 18, and 27 C. ference caused by the concentration difference. Due to the logarithmic relationship between concentration and potential from the Nernst equa- tion, a more reliable voltage reading can be obtained when the change in ion concentration exceeds ~10% of the initial concentration. Thus, when the same amounts of Na+ and Cl− are removed, a more obvious and reliable difference in the potential reading, and therefore a more ac- curate determination of the change in Na+ and Cl− concentrations, will be obtained when the initial concentration of the solution is low. There- fore, 0.06 M NaCl was used instead of 0.6 M NaCl for this experiment. We emphasize that all other experiments reported in this study were per- formed using 0.6 M NaCl as the electrolyte, and 0.06 M NaCl was used to calculate the desalination efficiency only to obtain more reliable results from the ion-selective electrodes. According to Le Chatelier’s principle (or if the Nernst equation is used to calculate the equilibrium potentials for the Na- and Cl-storage reactions), the Na- and Cl-storage reactions are more favored in 0.6 M NaCl than in 0.06 M NaCl. Thus, we expect that the desalination efficiency in 0.6 M NaCl will be at least as high as that determined using 0.06 M NaCl, which is shown below. Our results show that the FE of the Bi electrode for Cl− removal was ~100%, which agrees well with previous results [17,24,33,34]. The high FE of Bi for Cl− removal is attributed to the fact that there are no other oxidation reactions that can compete with the oxidation of Bi to BiOCl within the cell operating potential region [17]. The FE of the NaTi2(PO4)3 electrode for Na+ removal, on the other hand, was ~76%. The low FE is likely due to the occurrence of a redox reaction between Na3 Ti2 (PO4 )3 and O2 dissolved in the solution [32]. Overall, Charging Cell 1 was able to decrease the concentration of total dissolved solids (TDS) present in 0.06 M NaCl by ~15% after 27 C of charge were passed (Fig. 5b). We note that the NaTi2 (PO4 )3 /Bi cell removes Na+ and Cl− with a 3:1 ratio because Bi requires 3e− to remove one Cl− (Eq. 2) while NaTi2(PO4)3 requires 1e− to remove one Na+ (Eq. 1). Thus, when 100% of Na+ present in seawater has been removed by our desalination bat- tery, only 33% of Cl− will have been removed. The conversion of Bi to BiOCl also results in the production of protons (Eq. 2) [33]. In essence, our desalination cell can convert 0.6 M NaCl to 0.4 M HCl, reducing the ion concentration by 33% with a minimal energy input. We envision that the remaining Cl− and H+ can be removed by a successive electro- chemical process where Cl− is converted to Cl2 gas at the anode and H+ is converted to H2 gas at the cathode. These reactions are already used for the well-established chlor-alkali process to produce valuable Cl2 and H2 gases [43]. Thus, the construction of a cell to remove the re- maining Cl− and H+ should not require significant development efforts. In the chlor-alkali process, a highly concentrated NaCl solution (e.g. 4 M NaCl) is used as the feedwater and Cl− and H+ are removed in a 1:1 ratio to produce a concentrated NaOH solution. In our proposed pro- cess, 0.4 M HCl will be used as the feedwater and will be converted to slightly acidic brackish water. The combination of Charging Cell 1 with subsequent conversion of the product water to mildly acidic brackish water can offer an inexpensive route to convert seawater to brackish water, which then can be converted to fresh water by reverse osmosis (RO) or electrodialysis. (RO or electrodialysis will be used to remove the remaining Cl− and other ions present in the desalinated water). When brackish water is used as the feedwater, the energy and cost for fresh- water production by RO is significantly lower than when seawater is used as the feedwater. For example, the annual electricity cost required to operate a seawater RO (SWRO) plant is more than three times higher than that required for a brackish water (BWRO) plant of the same size [44]. Furthermore, even the construction costs of these two plants are drastically different; an SWRO plant costs more than twice as much to build as a BWRO plant of the same size [44]. Additionally, while the de- salination of seawater by electrodialysis is cost-prohibitive, desalination of brackish water by electrodialysis is cost-effective [36]. Thus, we believe that the dual-purpose ARNB demonstrated in this study, which can convert seawater to brackish water with a drastically reduced energy input, will be able to provide more efficient and practi- cal strategies for renewable electricity storage and desalination of sea- water. 3. Conclusions In summary, we have successfully demonstrated the construction of a dual-purpose ESS. The discharging cell used in this system was a high- voltage ARNB composed of a sodiated NaTi2 (PO4 )3 anode and a desodi- ated NiHCF cathode operating in 0.6 M NaCl. The discharging cell gen- erated an average output voltage (V) of 1.19 V, which is very close to the thermodynamic voltage limit of aqueous batteries (1.23 V). The charg- ing process was achieved in two separate cells, where each of the Na- storage electrodes was paired with a Bi/BiOCl electrode. The charging cell composed of a desodiated NaTi2(PO4)3 cathode and a Bi anode also achieved desalination during charging. As the removal of Na+ and Cl− in the desalination charging cell occurred through ion-specific electrode reactions, this cell did not require the use of a membrane. The Faradaic efficiencies for Na+ and Cl− removal in the desalination charging cell were ~76% and ~100%, respectively. The charging cell composed of a sodiated NiHCF anode and a BiOCl cathode performed salination dur- ing the charging process, regenerating the NiHCF and Bi electrodes for subsequent use in the discharging cell and desalination charging cell, respectively. The energy output of the discharging cell was found to be ~94% of the sum of the energy inputs required for the two charging cells. As the energy consumed for the desalination and salination pro- cesses is not truly consumed but rather stored in the system through the charging process, and the majority of the energy stored during charging is recovered during discharging, the extra energy consumed for desalina- 563PDF Image | seawater battery with desalination capabilities
PDF Search Title:
seawater battery with desalination capabilitiesOriginal File Name Searched:
10279292.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |