
PDF Publication Title:
Text from PDF Page: 007
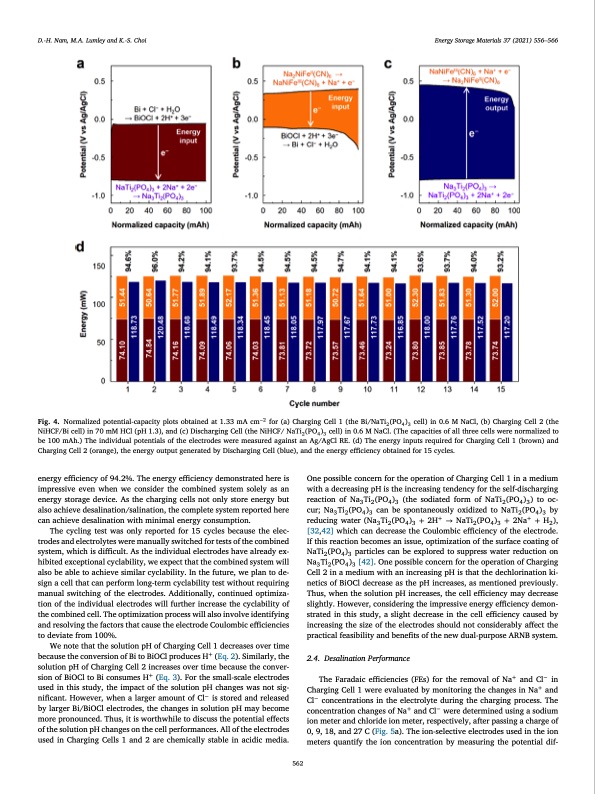
D.-H. Nam, M.A. Lumley and K.-S. Choi Energy Storage Materials 37 (2021) 556–566 Fig. 4. Normalized potential-capacity plots obtained at 1.33 mA cm−2 for (a) Charging Cell 1 (the Bi/NaTi2(PO4)3 cell) in 0.6 M NaCl, (b) Charging Cell 2 (the NiHCF/Bi cell) in 70 mM HCl (pH 1.3), and (c) Discharging Cell (the NiHCF/ NaTi2(PO4)3 cell) in 0.6 M NaCl. (The capacities of all three cells were normalized to be 100 mAh.) The individual potentials of the electrodes were measured against an Ag/AgCl RE. (d) The energy inputs required for Charging Cell 1 (brown) and Charging Cell 2 (orange), the energy output generated by Discharging Cell (blue), and the energy efficiency obtained for 15 cycles. energy efficiency of 94.2%. The energy efficiency demonstrated here is impressive even when we consider the combined system solely as an energy storage device. As the charging cells not only store energy but also achieve desalination/salination, the complete system reported here can achieve desalination with minimal energy consumption. The cycling test was only reported for 15 cycles because the elec- trodes and electrolytes were manually switched for tests of the combined system, which is difficult. As the individual electrodes have already ex- hibited exceptional cyclability, we expect that the combined system will also be able to achieve similar cyclability. In the future, we plan to de- sign a cell that can perform long-term cyclability test without requiring manual switching of the electrodes. Additionally, continued optimiza- tion of the individual electrodes will further increase the cyclability of the combined cell. The optimization process will also involve identifying and resolving the factors that cause the electrode Coulombic efficiencies to deviate from 100%. We note that the solution pH of Charging Cell 1 decreases over time because the conversion of Bi to BiOCl produces H+ (Eq. 2). Similarly, the solution pH of Charging Cell 2 increases over time because the conver- sion of BiOCl to Bi consumes H+ (Eq. 3). For the small-scale electrodes used in this study, the impact of the solution pH changes was not sig- nificant. However, when a larger amount of Cl− is stored and released by larger Bi/BiOCl electrodes, the changes in solution pH may become more pronounced. Thus, it is worthwhile to discuss the potential effects of the solution pH changes on the cell performances. All of the electrodes used in Charging Cells 1 and 2 are chemically stable in acidic media. One possible concern for the operation of Charging Cell 1 in a medium with a decreasing pH is the increasing tendency for the self-discharging reaction of Na3 Ti2 (PO4 )3 (the sodiated form of NaTi2 (PO4 )3 ) to oc- cur; Na3 Ti2 (PO4 )3 can be spontaneously oxidized to NaTi2 (PO4 )3 by reducing water (Na3 Ti2 (PO4 )3 + 2H+ → NaTi2 (PO4 )3 + 2Na+ + H2 ), [32,42] which can decrease the Coulombic efficiency of the electrode. If this reaction becomes an issue, optimization of the surface coating of NaTi2 (PO4 )3 particles can be explored to suppress water reduction on Na3 Ti2 (PO4 )3 [42]. One possible concern for the operation of Charging Cell 2 in a medium with an increasing pH is that the dechlorination ki- netics of BiOCl decrease as the pH increases, as mentioned previously. Thus, when the solution pH increases, the cell efficiency may decrease slightly. However, considering the impressive energy efficiency demon- strated in this study, a slight decrease in the cell efficiency caused by increasing the size of the electrodes should not considerably affect the practical feasibility and benefits of the new dual-purpose ARNB system. 2.4. Desalination Performance The Faradaic efficiencies (FEs) for the removal of Na+ and Cl− in Charging Cell 1 were evaluated by monitoring the changes in Na+ and Cl− concentrations in the electrolyte during the charging process. The concentration changes of Na+ and Cl− were determined using a sodium ion meter and chloride ion meter, respectively, after passing a charge of 0, 9, 18, and 27 C (Fig. 5a). The ion-selective electrodes used in the ion meters quantify the ion concentration by measuring the potential dif- 562PDF Image | seawater battery with desalination capabilities
PDF Search Title:
seawater battery with desalination capabilitiesOriginal File Name Searched:
10279292.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |