
PDF Publication Title:
Text from PDF Page: 011
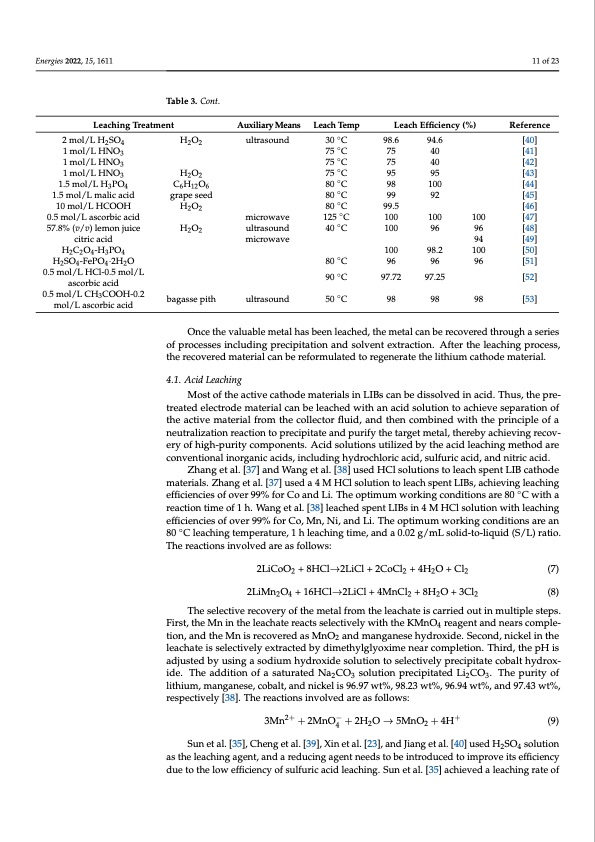
Energies 2022, 15, 1611 11 of 23 Table 3. Cont. Leaching Treatment Auxiliary Means ultrasound microwave ultrasound microwave ultrasound Leach Temp 30◦C 75◦C 75◦C 75◦C 80◦C 80◦C 80 ◦C 125 ◦C 40 ◦C Leach Efficiency (%) 98.6 94.6 75 40 75 40 95 95 98 100 99 92 99.5 100 100 100 96 2 mol/L H2SO4 1 mol/L HNO3 1 mol/L HNO3 1 mol/L HNO3 1.5 mol/L H3PO4 1.5 mol/L malic acid 10 mol/L HCOOH 0.5 mol/L ascorbic acid 57.8% (v/v) lemon juice citric acid H2C2O4-H3PO4 H2 SO4 -FePO4 ·2H2 O 0.5 mol/L HCl-0.5 mol/L ascorbic acid 0.5 mol/L CH3COOH-0.2 mol/L ascorbic acid H2O2 H2O2 C6H12O6 grape seed H2O2 H2O2 bagasse pith Reference [40] [41] [42] [43] [44] [45] [46] 100 [47] 96 [48] 94 [49] 100 [50] 90 ◦C 97.72 97.25 [52] 50◦C 98 98 98 [53] 100 98.2 80◦C 96 96 96 [51] Once the valuable metal has been leached, the metal can be recovered through a series of processes including precipitation and solvent extraction. After the leaching process, the recovered material can be reformulated to regenerate the lithium cathode material. 4.1. Acid Leaching Most of the active cathode materials in LIBs can be dissolved in acid. Thus, the pre- treated electrode material can be leached with an acid solution to achieve separation of the active material from the collector fluid, and then combined with the principle of a neutralization reaction to precipitate and purify the target metal, thereby achieving recov- ery of high-purity components. Acid solutions utilized by the acid leaching method are conventional inorganic acids, including hydrochloric acid, sulfuric acid, and nitric acid. Zhang et al. [37] and Wang et al. [38] used HCl solutions to leach spent LIB cathode materials. Zhang et al. [37] used a 4 M HCl solution to leach spent LIBs, achieving leaching efficiencies of over 99% for Co and Li. The optimum working conditions are 80 ◦C with a reaction time of 1 h. Wang et al. [38] leached spent LIBs in 4 M HCl solution with leaching efficiencies of over 99% for Co, Mn, Ni, and Li. The optimum working conditions are an 80 ◦C leaching temperature, 1 h leaching time, and a 0.02 g/mL solid-to-liquid (S/L) ratio. The reactions involved are as follows: 2LiCoO2 + 8HCl→2LiCl + 2CoCl2 + 4H2O + Cl2 (7) 2LiMn2O4 + 16HCl→2LiCl + 4MnCl2 + 8H2O + 3Cl2 (8) The selective recovery of the metal from the leachate is carried out in multiple steps. First, the Mn in the leachate reacts selectively with the KMnO4 reagent and nears comple- tion, and the Mn is recovered as MnO2 and manganese hydroxide. Second, nickel in the leachate is selectively extracted by dimethylglyoxime near completion. Third, the pH is adjusted by using a sodium hydroxide solution to selectively precipitate cobalt hydrox- ide. The addition of a saturated Na2CO3 solution precipitated Li2CO3. The purity of lithium, manganese, cobalt, and nickel is 96.97 wt%, 98.23 wt%, 96.94 wt%, and 97.43 wt%, respectively [38]. The reactions involved are as follows: 3Mn2+ + 2MnO4− + 2H2O → 5MnO2 + 4H+ (9) Sun et al. [35], Cheng et al. [39], Xin et al. [23], and Jiang et al. [40] used H2SO4 solution as the leaching agent, and a reducing agent needs to be introduced to improve its efficiency due to the low efficiency of sulfuric acid leaching. Sun et al. [35] achieved a leaching rate ofPDF Image | Recycling of Lithium Batteries
PDF Search Title:
Recycling of Lithium BatteriesOriginal File Name Searched:
energies-15-01611.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |