
PDF Publication Title:
Text from PDF Page: 016
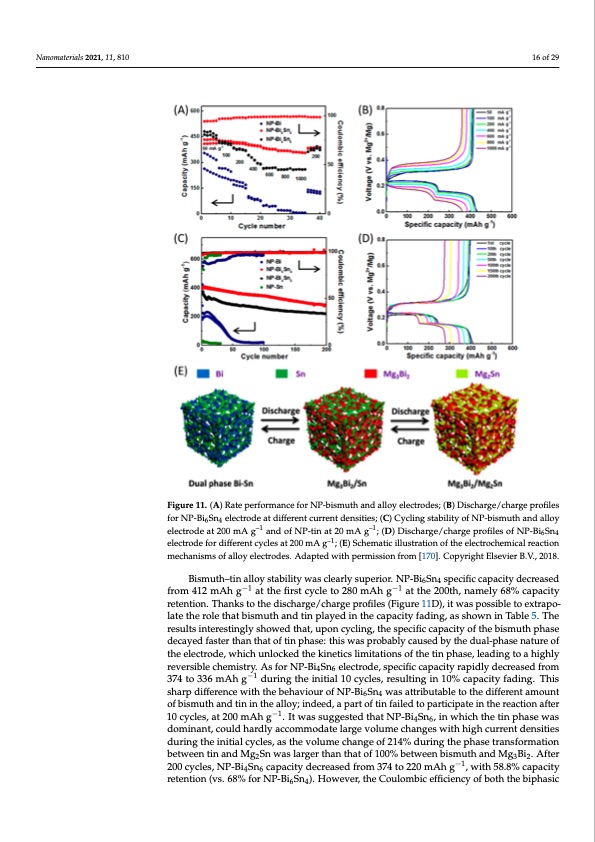
Nanomaterials 2021, 11, 810 Nanomaterials 2021, 11, x 16 of 29 15 of 28 Figure 11. (A) Rate performance for NP-bismuth and alloy electrodes; (B) Discharge/charge Figure 11. (A) Rate performance for NP-bismuth and alloy electrodes; (B) Discharge/charge profiles profiles for NP-Bi6Sn4 electrode at different current densities; (C) Cycling stability of NP-bismuth for NP-Bi6Sn4 electrode at different current densities; (C) Cycling stability of NP-bismuth and alloy and alloy electrode at 200 mA g–1 and of NP-tin at 20 mA g–1; (D) Discharge/charge profiles of NP- electrode at 200 mA g–1 and of NP-tin at 20 mA g–1; (D) Discharge/charge profiles of NP-Bi6Sn4 Bi6Sn4 electrode for different cycles at 200 mA g–1; (E) Schematic illustration of the electrochemical electrode for different cycles at 200 mA g–1; (E) Schematic illustration of the electrochemical reaction reaction mechanisms of alloy electrodes. Adapted with permission from [170]. Copyright Elsevier mechanisms of alloy electrodes. Adapted with permission from [170]. Copyright Elsevier B.V., 2018. B.V., 2018. Bismuth–tin alloy stability was clearly superior. NP-Bi6Sn4 specific capacity decreased Bismuth–tin alloy stability was clearly superior. NP-Bi6Sn4 specific capacity de- from 412 mAh g−1 at the first cycle to 280 mAh g−1 at the 200th, namely 68% capacity creased from 412 mAh g−1 at the first cycle to 280 mAh g−1 at the 200th, namely 68% capac- retention. Thanks to the discharge/charge profiles (Figure 11D), it was possible to extrapo- ity retention. Thanks to the discharge/charge profiles (Figure 11D), it was possible to ex- late the role that bismuth and tin played in the capacity fading, as shown in Table 5. The trapolate the role that bismuth and tin played in the capacity fading, as shown in Table 5. results interestingly showed that, upon cycling, the specific capacity of the bismuth phase The results interestingly showed that, upon cycling, the specific capacity of the bismuth decayed faster than that of tin phase: this was probably caused by the dual-phase nature of phase decayed faster than that of tin phase: this was probably caused by the dual-phase the electrode, which unlocked the kinetics limitations of the tin phase, leading to a highly nature of the electrode, which unlocked the kinetics limitations of the tin phase, leading reversible chemistry. As for NP-Bi4Sn6 electrode, specific capacity rapidly decreased from to a highly reversible chemistry. As for NP-Bi4Sn6 electrode, specific capacity rapidly de- 374 to 336 mAh g−1 during the initial 10 cycles, resulting in 10% capacity fading. This creased from 374 to 336 mAh g−1 during the initial 10 cycles, resulting in 10% capacity sharp difference with the behaviour of NP-Bi6Sn4 was attributable to the different amount fading. This sharp difference with the behaviour of NP-Bi6Sn4 was attributable to the dif- of bismuth and tin in the alloy; indeed, a part of tin failed to participate in the reaction after ferent amount of bismu−t1h and tin in the alloy; indeed, a part of tin failed to participate in 10 cycles, at 200 mAh g . It was suggested that NP-Bi4Sn6, in which the tin phase was the reaction after 10 cycles, at 200 mAh g−1. It was suggested that NP-Bi4Sn6, in which the dominant, could hardly accommodate large volume changes with high current densities tin phase was dominant, could hardly accommodate large volume changes with high cur- during the initial cycles, as the volume change of 214% during the phase transformation rent densities during the initial cycles, as the volume change of 214% during the phase between tin and Mg2Sn was larger than that of 100% between bismuth and Mg3Bi2. After transformation between tin and Mg2Sn was larger than that of 10−0%1 between bismuth and 200 cycles, NP-Bi4Sn6 capacity decreased from 374 to 220 mAh g , with 58.8% capacity Mg3Bi2. After 200 cycles, NP-Bi4Sn6 capacity decreased from 374 to 220 mAh g−1, with retention (vs. 68% for NP-Bi6Sn4). However, the Coulombic efficiency of both the biphasic 58.8% capacity retention (vs. 68% for NP-Bi6Sn4). However, the Coulombic efficiency ofPDF Image | Overview on Anodes for Magnesium Batteries
PDF Search Title:
Overview on Anodes for Magnesium BatteriesOriginal File Name Searched:
nanomaterials-11-00810.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |