
PDF Publication Title:
Text from PDF Page: 007
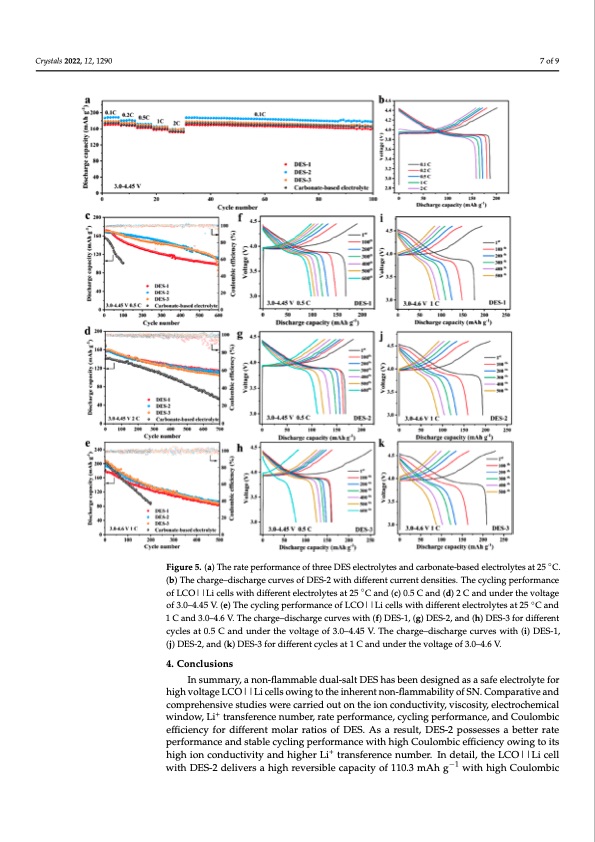
Crystals 2022, 12, x FOR PEER REVIEW 7 of 10 Crystals 2022, 12, 1290 7 of 9 Figure 5. (a) The rate performance of three DES electrolytes and carbonate-based electrolytes at 2◦5 Figure 5. (a) The rate performance of three DES electrolytes and carbonate-based electrolytes at 25 C. °C. (b) The charge–discharge curves of DES-2 with different current densities. The cycling perfor- (b) The charge–discharge curves of DES-2 with different current densities. The cycling performance mance of LCO||Li cells with different electrolytes at 25 °C and (c) 0.5 C and (d) 2 C and under the of LCO||Li cells with different electrolytes at 25 ◦C and (c) 0.5 C and (d) 2 C and under the voltage voltage of 3.0–4.45 V. (e) The cycling performance of LCO||Li cells with different electrolytes at 25 of 3.0–4.45 V. (e) The cycling performance of LCO||Li cells with different electrolytes at 25 ◦C and °C and 1 C and 3.0–4.6 V. The charge–discharge curves with (f) DES-1, (g) DES-2, and (h) DES-3 for 1 C and 3.0–4.6 V. The charge–discharge curves with (f) DES-1, (g) DES-2, and (h) DES-3 for different different cycles at 0.5 C and under the voltage of 3.0–4.45 V. The charge–discharge curves with (i) cycles at 0.5 C and under the voltage of 3.0–4.45 V. The charge–discharge curves with (i) DES-1, DES-1, (j) DES-2, and (k) DES-3 for different cycles at 1 C and under the voltage of 3.0–4.6 V. (j) DES-2, and (k) DES-3 for different cycles at 1 C and under the voltage of 3.0–4.6 V. The long cycling performance of DES electrolytes was further evaluated in LCO||Li cells under 3.0–4.45 V and 3.0–4.6 V. As shown in Figure 5c, the cell with DES-2 delivers Insummary,anon-flamm−a1bledual-saltDEShasbeendesignedasasafeelectrolytefor a high capacity of 111.5 mAh g after 600 cycles at 0.5 C under 3.0–4.45 V, which is higher high voltage LCO||Li cell−s1 owing to the inherent non-flammability of SN. Comparative and than DES-1 of 97.9 mAh g and DES-3 of 77.5 mAh g−1. In contrast, carbonate-based elec- 4. Conclusions comprehensive studies were carried out on the ion conductivity, viscosity, electrochemical trolytes exhibit a relatively low initial capacity of 153.9 mAh g−1 and suffer from rapid window, Li+ transference number, rate performance, cycling performance, and Coulombic capacity decay. Figure 5f–h shows the voltage–capacity curve of three DES electrolytes efficiency for different molar ratios of DES. As a result, DES-2 possesses a better rate with different cycles under 3.0–4.45 V. The results demonstrate that the initial Coulombic performance and stable cycling performance with high Coulombic efficiency owing to its efficiencies of DES-1, DES-2, and DES-3 are 87.9%, 91.2%, and 63.1%, respectively. More- high ion conductivity and higher Li+ transference number. In detail, the LCO||Li cell over, DES-2 exhibits a lower polarization during cycling. Moreover, the cells display out- with DES-2 delivers a high reversible capacity of 110.3 mAh g−1 with high Coulombic standing cycling stability, with the reversible capacity of 112.1, 110.3, and 105.0 mAh g−1PDF Image | Non-Flammable Dual-Salt Deep Eutectic Electrolyte
PDF Search Title:
Non-Flammable Dual-Salt Deep Eutectic ElectrolyteOriginal File Name Searched:
crystals-12-01290-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |