
PDF Publication Title:
Text from PDF Page: 009
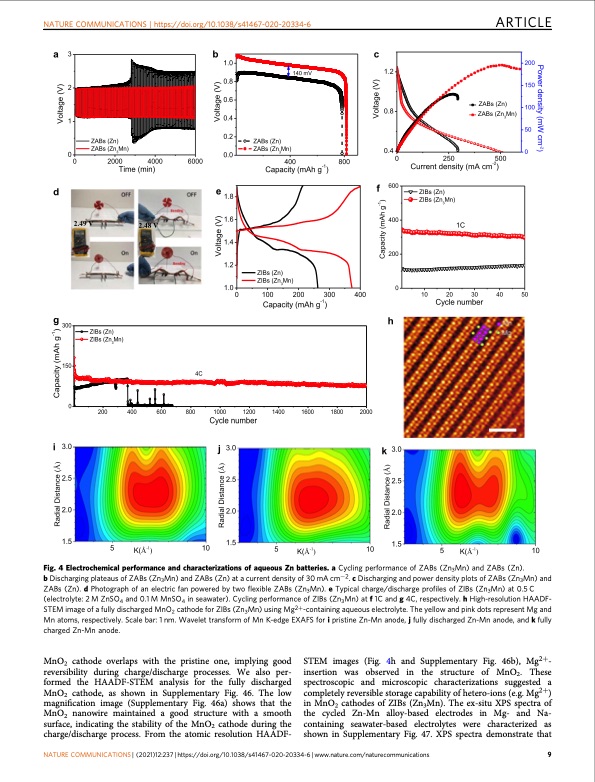
NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-020-20334-6 ARTICLE Fig. 4 Electrochemical performance and characterizations of aqueous Zn batteries. a Cycling performance of ZABs (Zn3Mn) and ZABs (Zn). b Discharging plateaus of ZABs (Zn3Mn) and ZABs (Zn) at a current density of 30 mA cm−2. c Discharging and power density plots of ZABs (Zn3Mn) and ZABs (Zn). d Photograph of an electric fan powered by two flexible ZABs (Zn3Mn). e Typical charge/discharge profiles of ZIBs (Zn3Mn) at 0.5 C (electrolyte: 2 M ZnSO4 and 0.1 M MnSO4 in seawater). Cycling performance of ZIBs (Zn3Mn) at f 1C and g 4C, respectively. h High-resolution HAADF- STEM image of a fully discharged MnO2 cathode for ZIBs (Zn3Mn) using Mg2+-containing aqueous electrolyte. The yellow and pink dots represent Mg and Mn atoms, respectively. Scale bar: 1 nm. Wavelet transform of Mn K-edge EXAFS for i pristine Zn-Mn anode, j fully discharged Zn-Mn anode, and k fully charged Zn-Mn anode. MnO2 cathode overlaps with the pristine one, implying good reversibility during charge/discharge processes. We also per- formed the HAADF-STEM analysis for the fully discharged MnO2 cathode, as shown in Supplementary Fig. 46. The low magnification image (Supplementary Fig. 46a) shows that the MnO2 nanowire maintained a good structure with a smooth surface, indicating the stability of the MnO2 cathode during the charge/discharge process. From the atomic resolution HAADF- STEM images (Fig. 4h and Supplementary Fig. 46b), Mg2+- insertion was observed in the structure of MnO2. These spectroscopic and microscopic characterizations suggested a completely reversible storage capability of hetero-ions (e.g. Mg2+) in MnO2 cathodes of ZIBs (Zn3Mn). The ex-situ XPS spectra of the cycled Zn-Mn alloy-based electrodes in Mg- and Na- containing seawater-based electrolytes were characterized as shown in Supplementary Fig. 47. XPS spectra demonstrate that NATURE COMMUNICATIONS | (2021)12:237 | https://doi.org/10.1038/s41467-020-20334-6 | www.nature.com/naturecommunications 9PDF Image | high-performance dendrite-free seawater-based batteries
PDF Search Title:
high-performance dendrite-free seawater-based batteriesOriginal File Name Searched:
s41467-020-20334-6.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |