
PDF Publication Title:
Text from PDF Page: 006
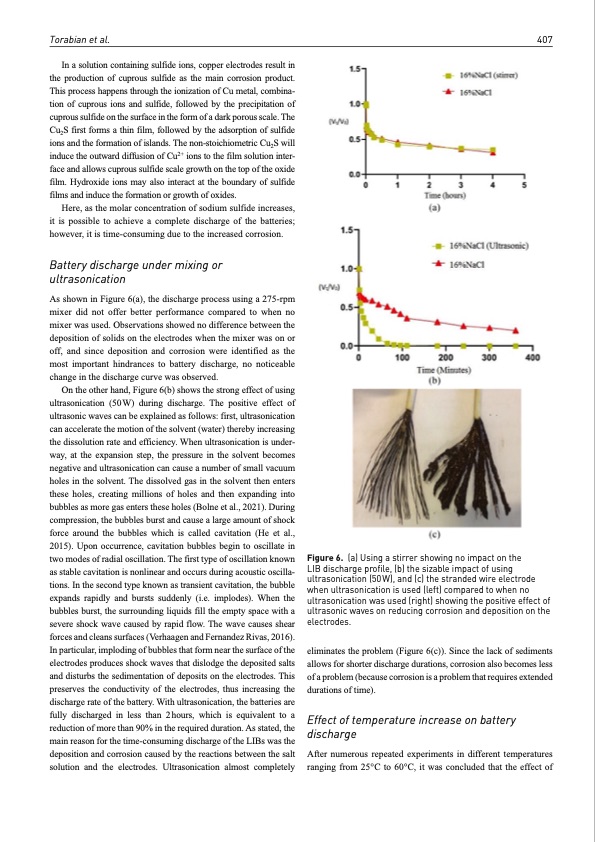
Torabian et al. 407 In a solution containing sulfide ions, copper electrodes result in the production of cuprous sulfide as the main corrosion product. This process happens through the ionization of Cu metal, combina- tion of cuprous ions and sulfide, followed by the precipitation of cuprous sulfide on the surface in the form of a dark porous scale. The Cu2S first forms a thin film, followed by the adsorption of sulfide ions and the formation of islands. The non-stoichiometric Cu2S will induce the outward diffusion of Cu2+ ions to the film solution inter- face and allows cuprous sulfide scale growth on the top of the oxide film. Hydroxide ions may also interact at the boundary of sulfide films and induce the formation or growth of oxides. Here, as the molar concentration of sodium sulfide increases, it is possible to achieve a complete discharge of the batteries; however, it is time-consuming due to the increased corrosion. Battery discharge under mixing or ultrasonication As shown in Figure 6(a), the discharge process using a 275-rpm mixer did not offer better performance compared to when no mixer was used. Observations showed no difference between the deposition of solids on the electrodes when the mixer was on or off, and since deposition and corrosion were identified as the most important hindrances to battery discharge, no noticeable change in the discharge curve was observed. On the other hand, Figure 6(b) shows the strong effect of using ultrasonication (50W) during discharge. The positive effect of ultrasonic waves can be explained as follows: first, ultrasonication can accelerate the motion of the solvent (water) thereby increasing the dissolution rate and efficiency. When ultrasonication is under- way, at the expansion step, the pressure in the solvent becomes negative and ultrasonication can cause a number of small vacuum holes in the solvent. The dissolved gas in the solvent then enters these holes, creating millions of holes and then expanding into bubbles as more gas enters these holes (Bolne et al., 2021). During compression, the bubbles burst and cause a large amount of shock force around the bubbles which is called cavitation (He et al., 2015). Upon occurrence, cavitation bubbles begin to oscillate in two modes of radial oscillation. The first type of oscillation known as stable cavitation is nonlinear and occurs during acoustic oscilla- tions. In the second type known as transient cavitation, the bubble expands rapidly and bursts suddenly (i.e. implodes). When the bubbles burst, the surrounding liquids fill the empty space with a severe shock wave caused by rapid flow. The wave causes shear forces and cleans surfaces (Verhaagen and Fernandez Rivas, 2016). In particular, imploding of bubbles that form near the surface of the electrodes produces shock waves that dislodge the deposited salts and disturbs the sedimentation of deposits on the electrodes. This preserves the conductivity of the electrodes, thus increasing the discharge rate of the battery. With ultrasonication, the batteries are fully discharged in less than 2hours, which is equivalent to a reduction of more than 90% in the required duration. As stated, the main reason for the time-consuming discharge of the LIBs was the deposition and corrosion caused by the reactions between the salt solution and the electrodes. Ultrasonication almost completely Figure 6. (a) Using a stirrer showing no impact on the LIB discharge profile, (b) the sizable impact of using ultrasonication (50 W), and (c) the stranded wire electrode when ultrasonication is used (left) compared to when no ultrasonication was used (right) showing the positive effect of ultrasonic waves on reducing corrosion and deposition on the electrodes. eliminates the problem (Figure 6(c)). Since the lack of sediments allows for shorter discharge durations, corrosion also becomes less of a problem (because corrosion is a problem that requires extended durations of time). Effect of temperature increase on battery discharge After numerous repeated experiments in different temperatures ranging from 25°C to 60°C, it was concluded that the effect ofPDF Image | Discharge of lithium-ion batteries in salt solutions
PDF Search Title:
Discharge of lithium-ion batteries in salt solutionsOriginal File Name Searched:
0734242x211022658.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |