
PDF Publication Title:
Text from PDF Page: 005
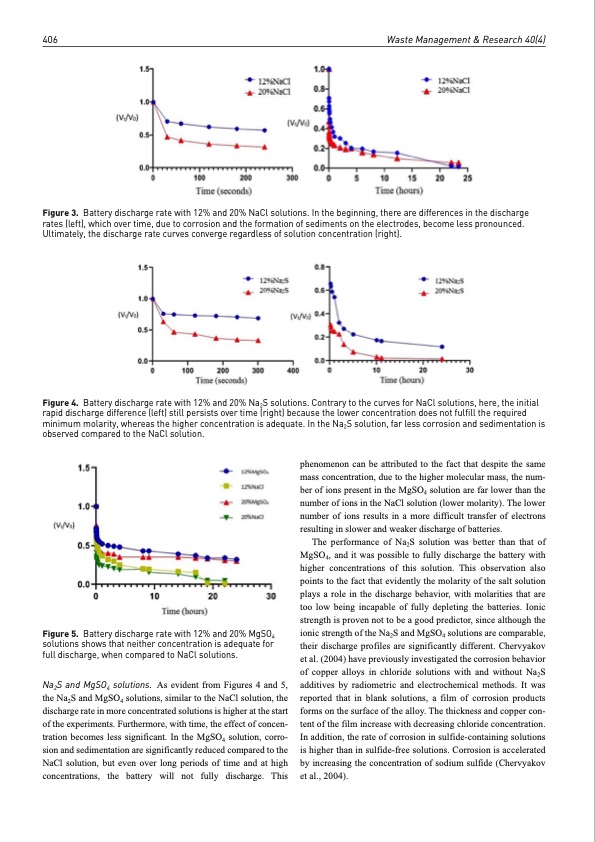
406 Waste Management & Research 40(4) Figure 3. Battery discharge rate with 12% and 20% NaCl solutions. In the beginning, there are differences in the discharge rates (left), which over time, due to corrosion and the formation of sediments on the electrodes, become less pronounced. Ultimately, the discharge rate curves converge regardless of solution concentration (right). Figure 4. Battery discharge rate with 12% and 20% Na2S solutions. Contrary to the curves for NaCl solutions, here, the initial rapid discharge difference (left) still persists over time (right) because the lower concentration does not fulfill the required minimum molarity, whereas the higher concentration is adequate. In the Na2S solution, far less corrosion and sedimentation is observed compared to the NaCl solution. Figure 5. Battery discharge rate with 12% and 20% MgSO4 solutions shows that neither concentration is adequate for full discharge, when compared to NaCl solutions. Na2S and MgSO4 solutions. As evident from Figures 4 and 5, the Na2S and MgSO4 solutions, similar to the NaCl solution, the discharge rate in more concentrated solutions is higher at the start of the experiments. Furthermore, with time, the effect of concen- tration becomes less significant. In the MgSO4 solution, corro- sion and sedimentation are significantly reduced compared to the NaCl solution, but even over long periods of time and at high concentrations, the battery will not fully discharge. This phenomenon can be attributed to the fact that despite the same mass concentration, due to the higher molecular mass, the num- ber of ions present in the MgSO4 solution are far lower than the number of ions in the NaCl solution (lower molarity). The lower number of ions results in a more difficult transfer of electrons resulting in slower and weaker discharge of batteries. The performance of Na2S solution was better than that of MgSO4, and it was possible to fully discharge the battery with higher concentrations of this solution. This observation also points to the fact that evidently the molarity of the salt solution plays a role in the discharge behavior, with molarities that are too low being incapable of fully depleting the batteries. Ionic strength is proven not to be a good predictor, since although the ionic strength of the Na2S and MgSO4 solutions are comparable, their discharge profiles are significantly different. Chervyakov et al. (2004) have previously investigated the corrosion behavior of copper alloys in chloride solutions with and without Na2S additives by radiometric and electrochemical methods. It was reported that in blank solutions, a film of corrosion products forms on the surface of the alloy. The thickness and copper con- tent of the film increase with decreasing chloride concentration. In addition, the rate of corrosion in sulfide-containing solutions is higher than in sulfide-free solutions. Corrosion is accelerated by increasing the concentration of sodium sulfide (Chervyakov etal.,2004).PDF Image | Discharge of lithium-ion batteries in salt solutions
PDF Search Title:
Discharge of lithium-ion batteries in salt solutionsOriginal File Name Searched:
0734242x211022658.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |