
PDF Publication Title:
Text from PDF Page: 012
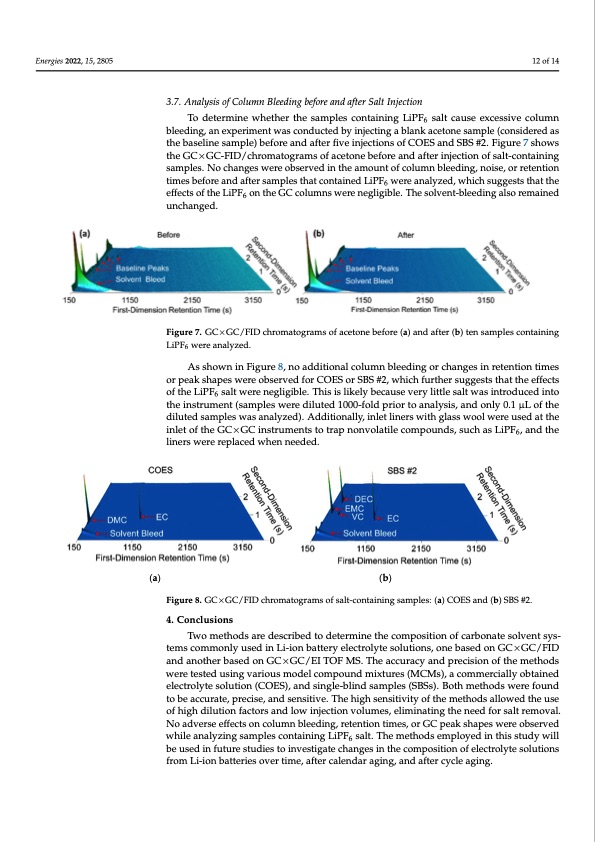
Energies 2022, 15, 2805 12 of 14 3.7. Analysis of Column Bleeding before and after Salt Injection To determine whether the samples containing LiPF6 salt cause excessive column bleeding, an experiment was conducted by injecting a blank acetone sample (considered as the baseline sample) before and after five injections of COES and SBS #2. Figure 7 shows the GC×GC-FID/chromatograms of acetone before and after injection of salt-containing samples. No changes were observed in the amount of column bleeding, noise, or retention times before and after samples that contained LiPF6 were analyzed, which suggests that the effects of the LiPF6 on the GC columns were negligible. The solvent-bleeding also remained Fuingcuhrean6.gVedol.ume percentages determined for carbonates in SBS #1 (a), SBS #2 (b), and COES (c). Figure 7. GC×GC/FID chromatograms of acetone before (a) and after (b) ten samples containing Figure 7. GC×GC/FID chromatograms of acetone before (a) and after (b) ten samples containing LiPF6 were analyzed. LiPF6 were analyzed. As shown in Figure 8, no additional column bleeding or changes in retention times As shown in Figure 8, no additional column bleeding or changes in retention times or peak shapes were observed for COES or SBS #2, which further suggests that the effects or peak shapes were observed for COES or SBS #2, which further suggests that the effects of the LiPF6 salt were negligible. This is likely because very little salt was introduced into of the LiPF6 salt were negligible. This is likely because very little salt was introduced into the instrument (samplles were diluted 1000--ffolld priiorr tto anallysiis,, and onlly 0..1 μL of the liners were replaced when needed. diluted samples was analyzed). Additionally, inlet liners with glass wool were used at the Energies 2022, 15, x FOR PEER REVIEW 13 of 14 inlet of the GC×GCiinssttrrumeenttsstto ttrap nonvollattiille compounds,, such as LiPF6,, and the 6 (a) (b) Figure 8. GC×GC/FID chromatograms of salt-containing samples: (a) COES and (b) SBS #2. Figure 8. GC×GC/FID chromatograms of salt-containing samples: (a) COES and (b) SBS #2. 44..CCoonncclulussioionnss TTwoomethodsareddeessccrriibbeedtotodedtetremrminienethtehceomcopmopsiotsioitnioonfcoafrbcaornbaotneastoelvseonlvtesnyts- styesmtesmcsocmomomnolynluysuesdedininLiL-iio-inonbabtatettreyryelelcetcrtorloyltyetesosloulutitoionns,so, onneebbaaseseddoonnGC×GC/FFIID aannddaannootthheerrbaasseedonGC×GGCC/E/IETIOTOFFMMS.ST. Theheacacucurarcaycyananddpprerecicsiisoionnooffththeemeetthhooddss weerreetteesstteedussininggvaarrioiouussmooddeellccoomppoouunnddmixixtuturreess((MCMss),),aaccoommeerrcciaiallylyoobbtatainineedd eelelecctrtroolylytetessoolulutitoionn(C(COEESS),),aannddssiningglele-b-blilninddssaamppleless((SSBBSSss).).BBooththmeeththooddssweerreeffoouunndd to be accurate, precise, and sensitive. The high sensitivity of the methods allowed the use to be accurate, precise, and sensitive. The high sensitivity of the methods allowed the use of high dilution factors and low injection volumes, eliminating the need for salt removal. of high dilution factors and low injection volumes, eliminating the need for salt removal. No adverse effects on column bleeding, retention times, or GC peak shapes were observed No adverse effects on column bleeding, retention times, or GC peak shapes were observed whileanalyzingsamplescontainingLiPF salt.Themethodsemployedinthisstudywill while analyzing samples containing LiPF66salt. The methods employed in this study will be used in future studies to investigate changes in the composition of electrolyte solutions be used in future studies to investigate changes in the composition of electrolyte solutions from Li-ion batteries over time, after calendar aging, and after cycle aging. from Li-ion batteries over time, after calendar aging, and after cycle aging. Author Contributions: Conceptualization, G.K. and J.K.O.; methodology, M.P., L.E.C.-M., B.A.M., H.I.K., G.K. and J.K.O.; formal analysis, M.P., L.E.C.-M. and B.A.M.; investigation, M.P., L.E.C.-M. and B.A.M.; resources, H.I.K., G.K. and J.K.O.; data curation, M.P., L.E.C.-M. and B.A.M.; writing— original draft preparation, M.P., L.E.C.-M. and B.A.M.; writing—review and editing, M.P., L.E.C.- M., B.A.M., H.I.K., G.K. and J.K.O.; visualization, M.P.; supervision, H.I.K., G.K. and J.K.O.; project administration, H.I.K., G.K. and J.K.O.; funding acquisition, G.K. and J.K.O. All authors have readPDF Image | Carbonate Solvent Systems Used in Lithium-Ion Batteries
PDF Search Title:
Carbonate Solvent Systems Used in Lithium-Ion BatteriesOriginal File Name Searched:
energies-15-02805.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |