
PDF Publication Title:
Text from PDF Page: 041
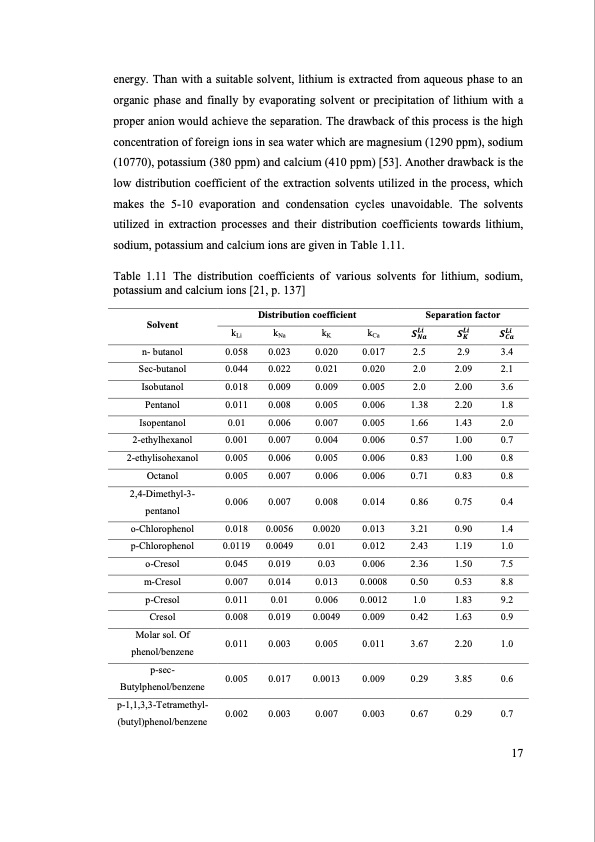
energy. Than with a suitable solvent, lithium is extracted from aqueous phase to an organic phase and finally by evaporating solvent or precipitation of lithium with a proper anion would achieve the separation. The drawback of this process is the high concentration of foreign ions in sea water which are magnesium (1290 ppm), sodium (10770), potassium (380 ppm) and calcium (410 ppm) [53]. Another drawback is the low distribution coefficient of the extraction solvents utilized in the process, which makes the 5-10 evaporation and condensation cycles unavoidable. The solvents utilized in extraction processes and their distribution coefficients towards lithium, sodium, potassium and calcium ions are given in Table 1.11. Table 1.11 The distribution coefficients of various solvents for lithium, sodium, potassium and calcium ions [21, p. 137] Solvent n- butanol Sec-butanol Isobutanol Pentanol Isopentanol 2-ethylhexanol 2-ethylisohexanol Octanol 2,4-Dimethyl-3- pentanol o-Chlorophenol p-Chlorophenol o-Cresol m-Cresol p-Cresol Cresol Molar sol. Of phenol/benzene p-sec- Butylphenol/benzene p-1,1,3,3-Tetramethyl- (butyl)phenol/benzene Distribution coefficient Separation factor k k k k 𝑺𝑳𝒊 𝑺𝑳𝒊 𝑺𝑳𝒊 Li Na K Ca 𝑵𝒂 𝑲 𝑪𝒂 0.058 0.023 0.044 0.022 0.018 0.009 0.011 0.008 0.01 0.006 0.001 0.007 0.005 0.006 0.005 0.007 0.006 0.007 0.018 0.0056 0.0119 0.0049 0.045 0.019 0.007 0.014 0.011 0.01 0.008 0.019 0.011 0.003 0.005 0.017 0.002 0.003 0.020 0.017 2.5 2.9 3.4 0.021 0.020 2.0 2.09 2.1 0.009 0.005 2.0 2.00 3.6 0.005 0.006 1.38 2.20 1.8 0.007 0.005 1.66 1.43 2.0 0.004 0.006 0.57 1.00 0.7 0.005 0.006 0.83 1.00 0.8 0.006 0.006 0.71 0.83 0.8 0.008 0.014 0.86 0.75 0.4 0.0020 0.013 3.21 0.90 1.4 0.01 0.012 2.43 1.19 1.0 0.03 0.006 2.36 1.50 7.5 0.013 0.0008 0.50 0.53 8.8 0.006 0.0012 1.0 1.83 9.2 0.0049 0.009 0.42 1.63 0.9 0.005 0.011 3.67 2.20 1.0 0.0013 0.009 0.29 3.85 0.6 0.007 0.003 0.67 0.29 0.7 17PDF Image | SEPARATION OF LITHIUM FROM BRINES
PDF Search Title:
SEPARATION OF LITHIUM FROM BRINESOriginal File Name Searched:
separation-lithium-from-brine.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |