
PDF Publication Title:
Text from PDF Page: 020
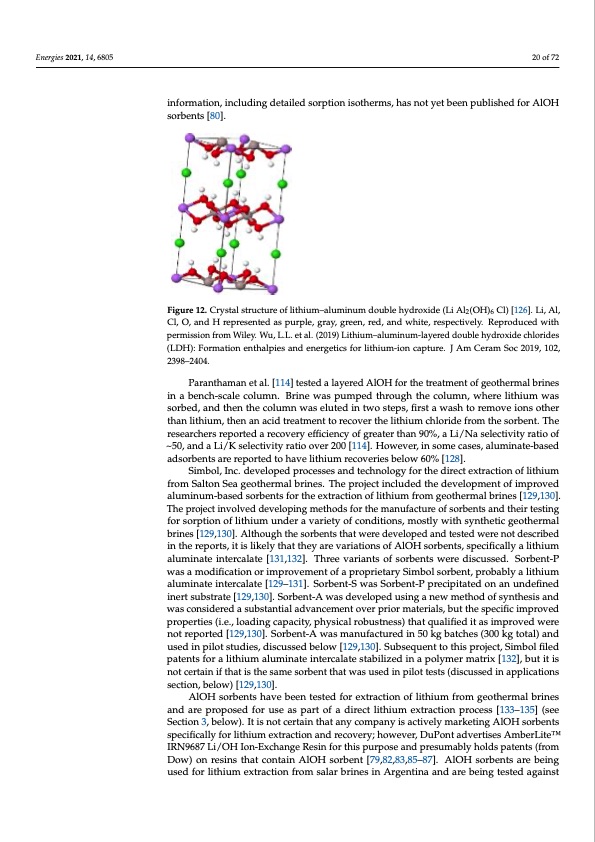
Energies 2021, 14, 6805 AlOH has been proposed for use as a lithium sorbent by a number of researchers; however, sorption capacities for AlOH sorbents are reported to be less than 8 mg/g [110,117,118,120]. One effective variant of AlOH sorbents are layered lithium-aluminum double hydroxides (Figure 12), which have been demonstrated as effective for removal of lithium for complex solutions. However, the stability of the sorbent for repeated sorption- extraction cycles may impact the practical application of this sorbent in[8fo0,r1m14a,t1io1n6,1i2n0c,l1u2d1i,n1g22d,1e2ta3i,l1e2d4,s1o2r5p,1ti2o6n,1i2s7o]t.heSromse, hfausnndoatmyenttbaeleinfpourbmliasthioend,foinrcAlulOdiHng sdorebtaeinletsd[s8o0r]p. tion isotherms, has not yet been published for AlOH sorbents [80]. Figure 12. Crystal structure of lithium–aluminum double hydroxide (Li Al2(OH)6 Cl) [126]. Li, Al, Figure 12. Crystal structure of lithium–aluminum double hydroxide (Li Al2(OH)6 Cl) [126]. Li, Al, Cl, O, and H represented as purple, gray, green, red, and white, respectively. Reproduced with Cl, O, and H represented as purple, gray, green, red, and white, respectively. Reproduced with permission from Wiley. Wu, L.L. et al. (2019) Lithium–aluminum-layered double hydroxide permission from Wiley. Wu, L.L. et al. (2019) Lithium–aluminum-layered double hydroxide chlorides chlorides (LDH): Formation enthalpies and energetics for lithium-ion capture. J Am Ceram Soc 2019, (LDH): Formation enthalpies and energetics for lithium-ion capture. J Am Ceram Soc 2019, 102, 20 of 72 102, 2398–2404. 2398–2404. Paranthaman et al. [114] tested a layered AlOH for the treatment of geothermal brines Paranthaman et al. [114] tested a layered AlOH for the treatment of geothermal brines in a bench-scale column. Brine was pumped through the column, where lithium was in a bench-scale column. Brine was pumped through the column, where lithium was sorbed, and then the column was eluted in two steps, first a wash to remove ions other sorbed, and then the column was eluted in two steps, first a wash to remove ions other than lithium, then an acid treatment to recover the lithium chloride from the sorbent. The than lithium, then an acid treatment to recover the lithium chloride from the sorbent. The researchers reported a recovery efficiency of greater than 90%, a Li/Na selectivity ratio of researchers reported a recovery efficiency of greater than 90%, a Li/Na selectivity ratio of ~50, and a Li/K selectivity ratio over 200 [114]. However, in some cases, aluminate-based ~50, and a Li/K selectivity ratio over 200 [114]. However, in some cases, aluminate-based adsorbents are reported to have lithium recoveries below 60% [128]. adsorbents are reported to have lithium recoveries below 60% [128]. Simbol, Inc. developed processes and technology for the direct extraction of lithium Simbol, Inc. developed processes and technology for the direct extraction of lithium from Salton Sea geothermal brines. The project included the development of improved from Salton Sea geothermal brines. The project included the development of improved aluminum-based sorbents for the extraction of lithium from geothermal brines [129,130]. aluminum-based sorbents for the extraction of lithium from geothermal brines [129,130]. The project involved developing methods for the manufacture of sorbents and their The project involved developing methods for the manufacture of sorbents and their testing testing for sorption of lithium under a variety of conditions, mostly with synthetic for sorption of lithium under a variety of conditions, mostly with synthetic geothermal geothermal brines [129,130]. Although the sorbents that were developed and tested were brines [129,130]. Although the sorbents that were developed and tested were not described not described in the reports, it is likely that they are variations of AlOH sorbents, in the reports, it is likely that they are variations of AlOH sorbents, specifically a lithium specifically a lithium aluminate intercalate [131,132]. Three variants of sorbents were aluminate intercalate [131,132]. Three variants of sorbents were discussed. Sorbent-P discussed. Sorbent-P was a modification or improvement of a proprietary Simbol sorbent, was a modification or improvement of a proprietary Simbol sorbent, probably a lithium probably a lithium aluminate intercalate [129,130,131]. Sorbent-S was Sorbent-P aluminate intercalate [129–131]. Sorbent-S was Sorbent-P precipitated on an undefined precipitated on an undefined inert substrate [129,130]. Sorbent-A was developed using a inert substrate [129,130]. Sorbent-A was developed using a new method of synthesis and wnaeswcomnseitdheordedoafssuybnsthaenstisalanddvawncaesmceontsoidverepdriaorsmubastetarinatlisa,lbaudtvthaencsepmeceinfitc iomvperopvreidor pmroapteritaiels, (bi.uet.,thloeasdpinecgifciacpimacpitryo,vpehdypsricoaplerotibeuss(tin.ee.,slso)atdhiantgqcuapliaficeitdy,itpahsyismicpalrorovbeudswtnerses) ntohtarteqpuoarltiefided[1i2t9a,s13im0]p.rSoovrebdenwt-eArewnoast rmepaonrutefadct[u12re9d,13in0]5.0Sokrgbebnat-cAhewsa(3s0m0akngutfoactatul)raenddin u5s0edkign pbialtocthsetsud(3ie0s0, dkigscutostsaeld) baenldowu[s1e2d9,1in30p].ilSoutbsteuqduiens,t tdoisthcuissperdojebcetl,oSwimb[1o2l9fi,1le3d0]. pSautebnsetsqufoernat ltiothituhmis aplruomjeicnt,atSeiminbteorlcaflialetedsptabteilniztsedfoinr aploitlhyimumer malautmrixin[a1t3e2]i,nbteurtcaitlaiste nsottabceilritzaeidnifnthaaptoislythmeesramaetsroixrb[e1n3t2]t,hbatuwtiatsisusneodticnerptialointtiefsthsa(tdisctuhsesesdamineaspoprlbiceanttiotnhsat swecatisouns,ebdelionwp)il[o1t29te,1st3s0(]d. iscussed in applications section, below) [129,130]. AlOH sorbents have been tested for extraction of lithium from geothermal brines and are proposed for use as part of a direct lithium extraction process [133–135] (see Section 3, below). It is not certain that any company is actively marketing AlOH sorbents specifically for lithium extraction and recovery; however, DuPont advertises AmberLiteTM IRN9687 Li/OH Ion-Exchange Resin for this purpose and presumably holds patents (from Dow) on resins that contain AlOH sorbent [79,82,83,85–87]. AlOH sorbents are being used for lithium extraction from salar brines in Argentina and are being tested againstPDF Image | Recovery of Lithium from Geothermal Brines
PDF Search Title:
Recovery of Lithium from Geothermal BrinesOriginal File Name Searched:
energies-14-06805-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |