
PDF Publication Title:
Text from PDF Page: 019
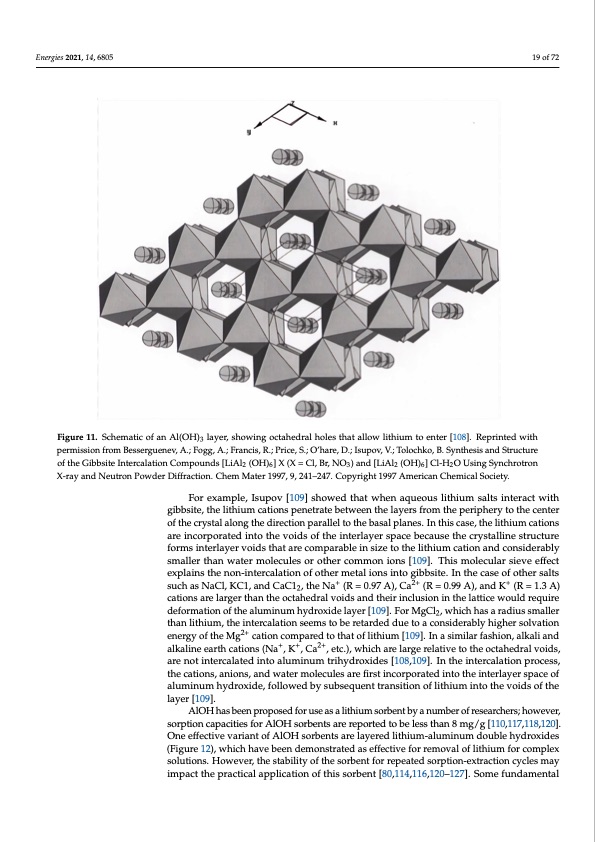
Energies 2021, 14, 6805 temperatures, crystalline hydrous aluminum oxides (AlOx) can be reacted directly with lithium salts to form crystalline lithium salt aluminates. Cations (lithium, magnesium, and transition metals) lie in the octahedral voids of the AlOH layers (Figure 11) [108,109,116]. As discussed in other sections, AlOH can be added to zeolite, resins, and other materials to make lithium sorbents [6,84,85,86,87,117,118,119]. Figure 11. Schematic of an Al(OH)3 layer, showing octahedral holes that allow lithium to enter [108]. Reprinted with Figure 11. Schematic of an Al(OH)3 layer, showing octahedral holes that allow lithium to enter [108]. Reprinted with Structure of the Gibbsite Intercalation Compounds [LiAl2 (OH)6] X (X = Cl, Br, NO3) and [LiAl2 (OH)6] Cl-H2O Using of the Gibbsite Intercalation Compounds [LiAl2 (OH)6] X (X = Cl, Br, NO3) and [LiAl2 (OH)6] Cl-H2O Using Synchrotron Synchrotron X-ray and Neutron Powder Diffraction. Chem Mater 1997, 9, 241–247. Copyright 1997 American Chemical X-ray and Neutron Powder Diffraction. Chem Mater 1997, 9, 241–247. Copyright 1997 American Chemical Society. For example, Isupov [109] showed that when aqueous lithium salts interact with For example, Isupov [109] showed that when aqueous lithium salts interact with Society. gibbsite, the lithium cations penetrate between the layers from the periphery to the center gibbsite, the lithium cations penetrate between the layers from the periphery to the center of the crystal along the direction parallel to the basal planes. In this case, the lithium cations 19 of 72 permission from Besserguenev, A.; Fogg, A.; Francis, R.; Price, S.; O'hare, D.; Isupov, V.; Tolochko, B. Synthesis and permission from Besserguenev, A.; Fogg, A.; Francis, R.; Price, S.; O’hare, D.; Isupov, V.; Tolochko, B. Synthesis and Structure of the crystal along the direction parallel to the basal planes. In this case, the lithium are incorporated into the voids of the interlayer space because the crystalline structure cations are incorporated into the voids of the interlayer space because the crystalline forms interlayer voids that are comparable in size to the lithium cation and considerably structure forms interlayer voids that are comparable in size to the lithium cation and smaller than water molecules or other common ions [109]. This molecular sieve effect considerably smaller than water molecules or other common ions [109]. This molecular explains the non-intercalation of other metal ions into gibbsite. In the case of other salts sieve effect explains the non-intercalation of other metal ions into gibbsite. In the case of suchasNaCl,KC1,andCaC12,theNa+ (R=0.97A),Ca2+ (R=0.99A),andK+ (R=1.3A) other salts such as NaCl, KC1, and CaC12, the Na+ (R = 0.97 A), Ca2+ (R = 0.99 A), and K+ (R cations are larger than the octahedral voids and their inclusion in the lattice would require = 1.3 A) cations are larger than the octahedral voids and their inclusion in the lattice would deformation of the aluminum hydroxide layer [109]. For MgCl2, which has a radius smaller require deformation of the aluminum hydroxide layer [109]. For MgCl2, which has a than lithium, the intercalation seems to be retarded due to a considerably higher solvation radius smaller th2a+n lithium, the intercalation seems to be retarded due to a considerably energy of the Mg cation compared to that of lithium [109]. In a similar fashion, alkali and 2+ higher solvation energy o+f th+e Mg2+ cation compared to that of lithium [109]. In a similar alkaline earth cations (Na , K , Ca , etc.), which are large relative to the octahedral voids, fashion, alkali and alkaline earth cations (Na+, K+, Ca2+, etc.), which are large relative to the are not intercalated into aluminum trihydroxides [108,109]. In the intercalation process, octahedral voids, are not intercalated into aluminum trihydroxides [108,109]. In the the cations, anions, and water molecules are first incorporated into the interlayer space of intercalation process, the cations, anions, and water molecules are first incorporated into aluminum hydroxide, followed by subsequent transition of lithium into the voids of the the interlayer space of aluminum hydroxide, followed by subsequent transition of lithium layer [109]. into the voids of the layer [109]. AlOH has been proposed for use as a lithium sorbent by a number of researchers; however, sorption capacities for AlOH sorbents are reported to be less than 8 mg/g [110,117,118,120]. One effective variant of AlOH sorbents are layered lithium-aluminum double hydroxides (Figure 12), which have been demonstrated as effective for removal of lithium for complex solutions. However, the stability of the sorbent for repeated sorption-extraction cycles may impact the practical application of this sorbent [80,114,116,120–127]. Some fundamentalPDF Image | Recovery of Lithium from Geothermal Brines
PDF Search Title:
Recovery of Lithium from Geothermal BrinesOriginal File Name Searched:
energies-14-06805-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |