
PDF Publication Title:
Text from PDF Page: 014
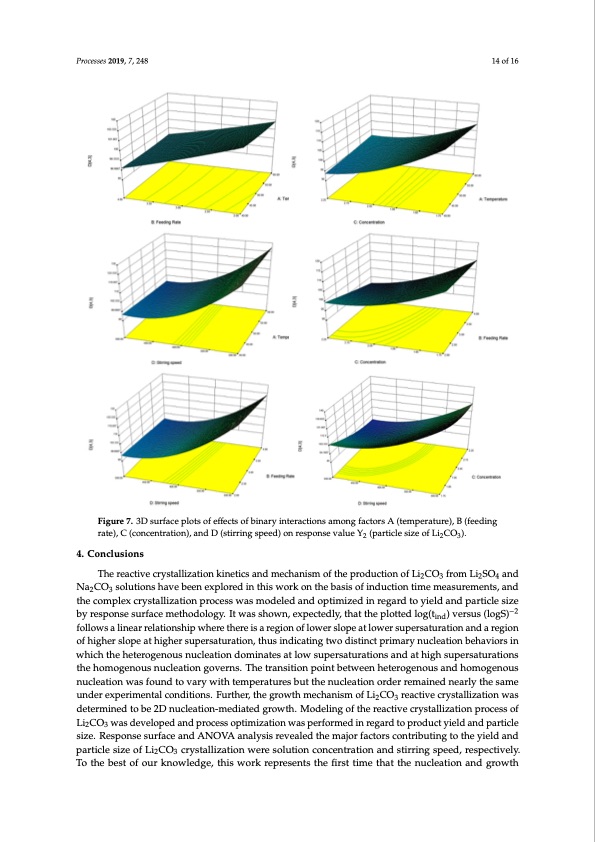
Processes 2019, 7, 248 14 of 16 Processes 2018, 6, x FOR PEER REVIEW 15 of 17 Figure 7. 3D surface plots of effects of binary interactions among factors A (temperature), B (feeding rate), C (concentration), and D (stirring speed) on response value Y2 (particle size of Li2CO3). Figure 7. 3D surface plots of effects of binary interactions among factors A (temperature), B (feeding rate), C (concentration), and D (stirring speed) on response value Y2 (particle size of Li2CO3). 4. Conclusions 4. CTohnecrlueasciotinvse crystallization kinetics and mechanism of the production of Li2CO3 from Li2SO4 and Na2CO3 solutions have been explored in this work on the basis of induction time measurements, and The reactive crystallization kinetics and mechanism of the production of Li2CO3 from Li2SO4 and the complex crystallization process was modeled and optimized in regard to yield and particle size Na2CO3 solutions have been explored in this work on the basis of induction time measurements, and by response surface methodology. It was shown, expectedly, that the plotted log(t ) versus (logS)−2 the complex crystallization process was modeled and optimized in regard to yieldianndd particle size follows a linear relationship where there is a region of lower slope at lower supersaturation and a reg−2ion by response surface methodology. It was shown, expectedly, that the plotted log(tind) versus (logS) of higher slope at higher supersaturation, thus indicating two distinct primary nucleation behaviors in follows a linear relationship where there is a region of lower slope at lower supersaturation and a whreicghiotnheofhhetigerhoegresnlopues antuhcliegahteironsudpoemrsiantuatreastioant ,lotwhusuipnedriscatiunrgattiwonosdainstdinact hpirgimh asurypenruscalteuartaiotnions thbeehoamviorgseninouwshnicuhcltehaetiohnetegrovgernosu.sTnhuectlreantisointiodnompoininatebsetawteloewnhseutpeerrosgaetunroautisonansdanhdomatohgiegnhous nusculpeaertisoantuwraatisofnosutnhdethoomvaorgyewnoiuthstneumclpeeartaiotnurgeosvbeurntsth.eThneuctrlaenatsiotinonorpdoeirntrebmetawineendhnetaerloygtehneousasme and homogenous nucleation was found to vary with temperatures but the nucleation order remained under experimental conditions. Further, the growth mechanism of Li2CO3 reactive crystallization was nearly the same under experimental conditions. Further, the growth mechanism of Li2CO3 reactive determined to be 2D nucleation-mediated growth. Modeling of the reactive crystallization process of crystallization was determined to be 2D nucleation-mediated growth. Modeling of the reactive Li2CO3 was developed and process optimization was performed in regard to product yield and particle crystallization process of Li2CO3 was developed and process optimization was performed in regard size. Response surface and ANOVA analysis revealed the major factors contributing to the yield and to product yield and particle size. Response surface and ANOVA analysis revealed the major factors particle size of Li2CO3 crystallization were solution concentration and stirring speed, respectively. To the best of our knowledge, this work represents the first time that the nucleation and growthPDF Image | Reactive Crystallization Process of Lithium Carbonate
PDF Search Title:
Reactive Crystallization Process of Lithium CarbonateOriginal File Name Searched:
processes-07-00248-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |