
PDF Publication Title:
Text from PDF Page: 018
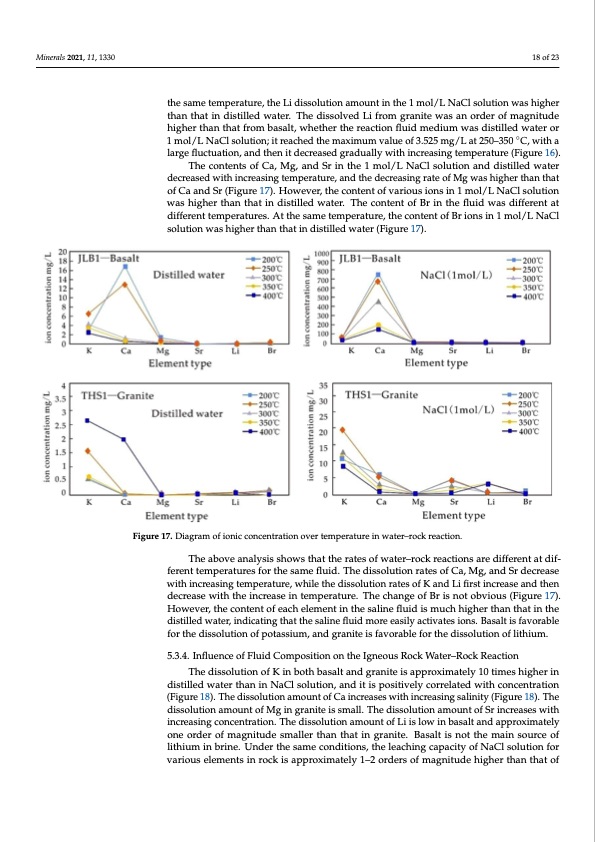
Minerals 2021, 11, 1330 18 of 23 Minerals 2021, 11, 1330 19 of 24 the same temperature, the Li dissolution amount in the 1 mol/L NaCl solution was higher than that in distilled water. The dissolved Li from granite was an order of magnitude higher than that from basalt, whether the reaction fluid medium was distilled water or 1 mol/L NaCl solution; it reached the maximum value of 3.525 mg/L at 250–350 ◦C, with a large fluctuation, and then it decreased gradually with increasing temperature (Figure 16). The contents of Ca, Mg, and Sr in the 1 mol/L NaCl solution and distilled water decreased with increasing temperature, and the decreasing rate of Mg was higher than that of Ca and Sr (Figure 17). However, the content of various ions in 1 mol/L NaCl solution was higher than that in distilled water. The content of Br in the fluid was different at different temperatures. At the same temperature, the content of Br ions in 1 mol/L NaCl solution was higher than that in distilled water (Figure 17). Figure 17. Diagram of ionic concentration over temperature in water–rock reaction. Figure 17. Diagram of ionic concentration over temperature in water–rock reaction. 5.3.4.TIhnfeluaebnocveoafnFalluyisdisCsohmowposstihtiaotnthoen rtahtesIgonfewouasteRr–orcokcWk raetaecrt–iRonocskarReedaciftfieornent at dif- ferent temperatures for the same fluid. The dissolution rates of Ca, Mg, and Sr decrease The dissolution of K in both basalt and granite is approximately 10 times higher in with increasing temperature, while the dissolution rates of K and Li first increase and then distilled water than in NaCl solution, and it is positively correlated with concentration decrease with the increase in temperature. The change of Br is not obvious (Figure 17). (Figure 18). The dissolution amount of Ca increases with increasing salinity (Figure 18). However, the content of each element in the saline fluid is much higher than that in the The dissolution amount of Mg in granite is small. The dissolution amount of Sr increases distilled water, indicating that the saline fluid more easily activates ions. Basalt is favorable with increasing concentration. The dissolution amount of Li is low in basalt and ap- for the dissolution of potassium, and granite is favorable for the dissolution of lithium. proximately one order of magnitude smaller than that in granite. Basalt is not the main source of lithium in brine. Under the same conditions, the leaching capacity of NaCl so- 5.3.4. Influence of Fluid Composition on the Igneous Rock Water–Rock Reaction lution for various elements in rock is approximately 1–2 orders of magnitude higher than The dissolution of K in both basalt and granite is approximately 10 times higher in that of deionized water (Figure 18), indicating that high-salinity fluid is the main migra- distilled water than in NaCl solution, and it is positively correlated with concentration tion carrier of ore-forming elements. (Figure 18). The dissolution amount of Ca increases with increasing salinity (Figure 18). The Compared with leaching results under normal temperatures and the chemical dissolution amount of Mg in granite is small. The dissolution amount of Sr increases with analysis results of Jianghan basin brine (Table 4), the dissolution of Li, K, Sr, Mg, and increasing concentration. The dissolution amount of Li is low in basalt and approximately other elements in autoclave water–rock reactions is significantly higher than the normal one order of magnitude smaller than that in granite. Basalt is not the main source of temperature immersion results, but still two orders of magnitude lower than the element lithium in brine. Under the same conditions, the leaching capacity of NaCl solution for content in the basin brine, indicating that high temperatures are more favorable for K, Li, various elements in rock is approximately 1–2 orders of magnitude higher than that of Na, and Sr enrichment. The process with higher-than-normal temperatures has a signif- icant impact on the composition of fluid, which is favorable for igneous rocks to react and provide material source for brine. Water–rock reaction of igneous rock is one of the im- portant processes for the mineralization of lithium–potassium-rich brine, and surface evaporation and concentration are the main mechanisms for the mineralization of brine.PDF Image | Origin of Lithium Potassium Rich Brines in the Jianghan
PDF Search Title:
Origin of Lithium Potassium Rich Brines in the JianghanOriginal File Name Searched:
minerals-11-01330-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |