
PDF Publication Title:
Text from PDF Page: 040
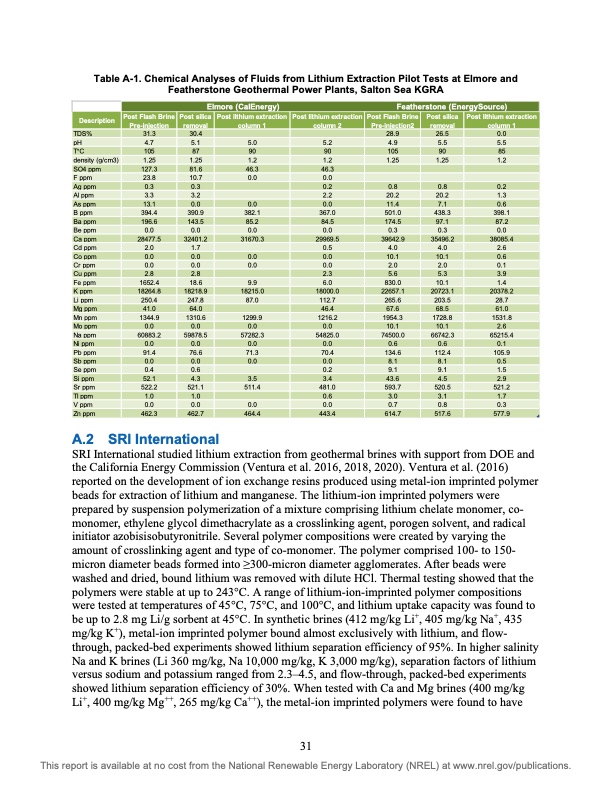
Table A-1. Chemical Analyses of Fluids from Lithium Extraction Pilot Tests at Elmore and Featherstone Geothermal Power Plants, Salton Sea KGRA TDS% pH T°C density (g/cm3) SO4 ppm F ppm Ag ppm Al ppm As ppm B ppm Ba ppm Be ppm Ca ppm Cd ppm Co ppm Cr ppm Cu ppm Fe ppm K ppm Li ppm Mg ppm Mn ppm Mo ppm Na ppm Ni ppm Pb ppm Sb ppm Se ppm Si ppm Sr ppm Tl ppm V ppm Zn ppm 31.3 4.7 105 1.25 127.3 23.8 0.3 3.3 13.1 394.4 196.6 0.0 28477.5 2.0 0.0 0.0 2.8 1652.4 18264.8 250.4 41.0 1344.9 0.0 60883.2 0.0 91.4 0.0 0.4 52.1 522.2 1.0 0.0 462.3 5.1 87 1.25 81.6 10.7 0.3 3.2 0.0 390.9 143.5 0.0 32401.2 1.7 0.0 0.0 2.8 18.6 18218.9 247.8 64.0 1310.6 0.0 59878.5 0.0 76.6 0.0 0.6 4.3 521.1 1.0 0.0 462.7 Elmore (CalEnergy) Featherstone (EnergySource) Description Post Flash Brine Post silica Post lithium extraction Post lithium extraction Post Flash Brine Post silica Post lithium extraction Pre-injection removal column 1 column 2 Pre-injection2 removal column 1 30.4 5.0 90 1.2 46.3 0.0 0.0 382.1 85.2 0.0 31670.3 0.0 0.0 9.9 18215.0 87.0 1299.9 0.0 57282.3 0.0 71.3 0.0 3.5 511.4 0.0 464.4 5.2 90 1.2 46.3 0.0 0.2 2.2 0.0 367.0 84.5 0.0 29969.5 0.5 0.0 0.0 2.3 6.0 18000.0 112.7 46.4 1216.2 0.0 54825.0 0.0 70.4 0.0 0.2 3.4 481.0 0.6 0.0 443.4 28.9 4.9 105 1.25 0.8 20.2 11.4 501.0 174.5 0.3 39642.9 4.0 10.1 2.0 5.6 830.0 22657.1 265.6 67.6 1954.3 10.1 74500.0 0.6 134.6 8.1 9.1 43.6 593.7 3.0 0.7 614.7 26.5 5.5 90 1.25 0.8 20.2 7.1 438.3 97.1 0.3 35496.2 4.0 10.1 2.0 5.3 10.1 20723.1 203.5 68.5 1728.8 10.1 66742.3 0.6 112.4 8.1 9.1 4.5 520.5 3.1 0.8 517.6 0.0 5.5 85 1.2 0.2 1.3 0.6 398.1 87.2 0.0 38085.4 2.6 0.6 0.1 3.9 1.4 20378.2 28.7 61.0 1531.8 2.6 65215.4 0.1 105.9 0.5 1.5 2.9 521.2 1.7 0.3 577.9 A.2 SRI International SRI International studied lithium extraction from geothermal brines with support from DOE and the California Energy Commission (Ventura et al. 2016, 2018, 2020). Ventura et al. (2016) reported on the development of ion exchange resins produced using metal-ion imprinted polymer beads for extraction of lithium and manganese. The lithium-ion imprinted polymers were prepared by suspension polymerization of a mixture comprising lithium chelate monomer, co- monomer, ethylene glycol dimethacrylate as a crosslinking agent, porogen solvent, and radical initiator azobisisobutyronitrile. Several polymer compositions were created by varying the amount of crosslinking agent and type of co-monomer. The polymer comprised 100- to 150- micron diameter beads formed into ≥300-micron diameter agglomerates. After beads were washed and dried, bound lithium was removed with dilute HCl. Thermal testing showed that the polymers were stable at up to 243°C. A range of lithium-ion-imprinted polymer compositions were tested at temperatures of 45°C, 75°C, and 100°C, and lithium uptake capacity was found to be up to 2.8 mg Li/g sorbent at 45°C. In synthetic brines (412 mg/kg Li+, 405 mg/kg Na+, 435 mg/kg K+), metal-ion imprinted polymer bound almost exclusively with lithium, and flow- through, packed-bed experiments showed lithium separation efficiency of 95%. In higher salinity Na and K brines (Li 360 mg/kg, Na 10,000 mg/kg, K 3,000 mg/kg), separation factors of lithium versus sodium and potassium ranged from 2.3–4.5, and flow-through, packed-bed experiments showed lithium separation efficiency of 30%. When tested with Ca and Mg brines (400 mg/kg Li+, 400 mg/kg Mg++, 265 mg/kg Ca++), the metal-ion imprinted polymers were found to have 31 This report is available at no cost from the National Renewable Energy Laboratory (NREL) at www.nrel.gov/publications.PDF Image | Lithium Extraction from Geothermal Brines
PDF Search Title:
Lithium Extraction from Geothermal BrinesOriginal File Name Searched:
79178.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |