
PDF Publication Title:
Text from PDF Page: 015
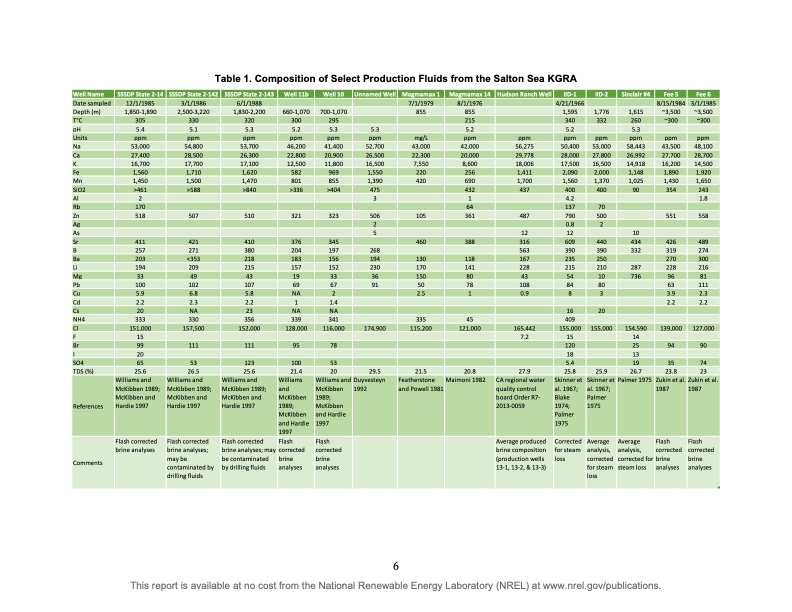
Well Name Date sampled Depth (m) T°C pH Units Na Ca K Fe Mn SiO2 Al Rb Zn Ag As Sr B Ba Li Mg Pb Cu Cd Cs NH4 Cl F Br I SO4 TDS (%) SSSDP State 2-14 SSSDP State 2-142 SSSDP State 2-143 Well 11b Well 10 Unnamed Well Magmamax 1 Magmamax 14 Hudson Ranch Well IID-1 IID-2 Sinclair #4 Fee 5 Fee 6 3/1/1985 ~3,500 ~300 ppm 48,100 28,700 14,500 1,920 1,650 243 1.8 558 489 274 300 216 81 111 2.3 2.2 127,000 90 74 23 Table 1. Composition of Select Production Fluids from the Salton Sea KGRA References Comments 12/1/1985 1,850-1,890 305 5.4 ppm 53,000 27,400 16,700 1,560 1,450 >461 2 170 518 411 257 203 194 33 100 5.9 2.2 20 333 151,000 15 99 20 65 25.6 Williams and McKibben 1989; McKibben and Hardie 1997 Flash corrected brine analyses 3/1/1986 2,500-3,220 330 5.1 ppm 54,800 28,500 17,700 1,710 1,500 >588 507 421 271 <353 209 49 102 6.8 2.3 NA 330 157,500 111 Williams and McKibben 1989; McKibben and Hardie 1997 53 26.5 Flash corrected brine analyses; may be contaminated by drilling fluids 6/1/1988 1,830-2,200 Williams and McKibben 1989; McKibben and Hardie 1997 320 5.3 ppm 53,700 26,300 17,100 1,620 1,470 >840 510 410 380 218 215 43 107 5.8 2.2 23 356 152,000 111 123 25.6 Flash corrected brine analyses; may be contaminated by drilling fluids 660-1,070 300 5.2 ppm 46,200 22,800 12,500 582 801 >336 321 376 204 183 157 19 69 NA 1 NA 339 128,000 95 100 21.4 Williams and McKibben 1989; McKibben and Hardie 1997 Flash corrected brine analyses 700-1,070 Flash corrected brine analyses 295 5.3 ppm 41,400 20,900 11,800 969 855 >404 323 345 197 156 152 33 67 2 1.4 NA 341 116,000 78 53 20 Williams and McKibben 1989; McKibben and Hardie 1997 5.3 ppm 52,700 26,500 16,500 1,550 1,390 475 3 506 2 5 268 194 230 36 91 174,900 29.5 Duyvesteyn 1992 7/1/1979 855 mg/L 43,000 22,300 7,550 220 420 105 460 130 170 150 50 2.5 335 115,200 21.5 Featherstone and Powell 1981 8/1/1976 855 215 5.2 ppm 42,000 20,000 8,600 256 690 432 1 64 361 388 118 141 80 78 1 45 121,000 20.8 Maimoni 1982 ppm 56,275 29,778 18,006 1,411 1,700 437 487 12 316 563 167 228 43 108 0.9 165,442 7.2 27.9 CA regional water quality control board Order R7- 2013-0059 Average produced brine composition (production wells 13-1, 13-2, & 13-3) 4/21/1966 1,595 340 5.2 ppm 50,400 28,000 17,500 2,090 1,560 400 4.2 137 790 0.8 12 609 390 235 215 54 84 8 16 409 155,000 15 120 18 5.4 25.8 Skinner et al. 1967; Blake 1974; Palmer 1975 Corrected for steam loss 1,776 332 ppm 53,000 27,800 16,500 2,000 1,370 400 70 500 2 440 390 250 210 10 80 3 20 155,000 25.9 Skinner et al. 1967; Palmer 1975 Average analysis, corrected for steam loss 1,615 260 5.3 ppm 58,443 26,992 14,918 1,148 1,025 90 10 434 332 287 736 154,590 14 25 13 19 26.7 Palmer 1975 Average analysis, corrected for steam loss 8/15/1984 ~3,500 ~300 ppm 43,500 27,700 16,200 1,890 1,430 354 551 426 319 270 228 96 63 3.9 2.2 139,000 94 35 23.8 Zukin et al. 1987 Flash corrected brine analyses Zukin et al. 1987 Flash corrected brine analyses 6 This report is available at no cost from the National Renewable Energy Laboratory (NREL) at www.nrel.gov/publications.PDF Image | Lithium Extraction from Geothermal Brines
PDF Search Title:
Lithium Extraction from Geothermal BrinesOriginal File Name Searched:
79178.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |