
PDF Publication Title:
Text from PDF Page: 019
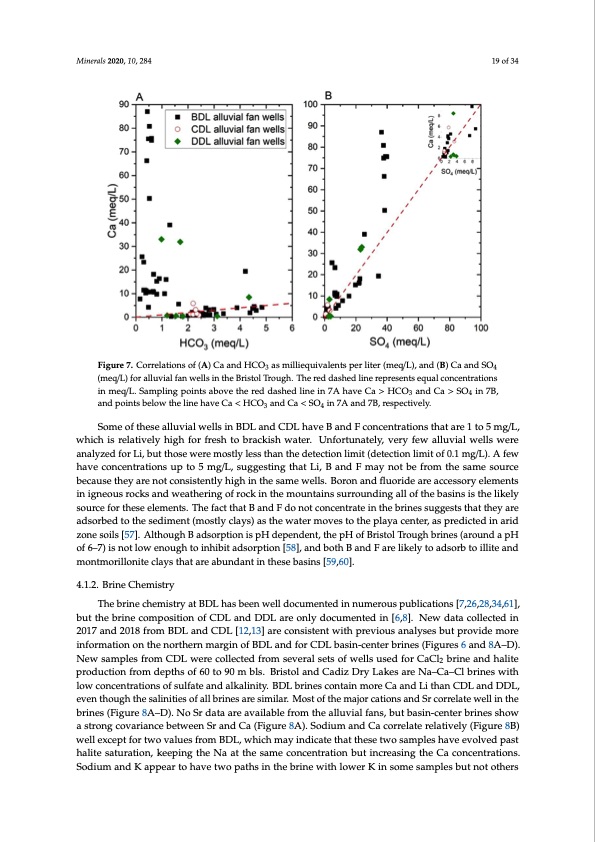
Minerals 2020, 10, x FOR PEER REVIEW 19 of 34 Minerals 2020, 10, 284 19 of 34 Figure 7. Correlations of (A) Ca and HCO3 as milliequivalents per liter (meq/L), and (B) Ca and SO4 Figure 7. Correlations of (A) Ca and HCO3 as milliequivalents per liter (meq/L), and (B) Ca and SO4 (meq/L) for alluvial fan wells in the Bristol Trough. The red dashed line represents equal concentrations (meq/L) for alluvial fan wells in the Bristol Trough. The red dashed line represents equal in meq/L. Sampling points above the red dashed line in 7A have Ca > HCO3 and Ca > SO4 in 7B, concentrations in meq/L. Sampling points above the red dashed line in 7A have Ca > HCO3 and Ca > and points below the line have Ca < HCO3 and Ca < SO4 in 7A and 7B, respectively. SO4 in 7B, and points below the line have Ca < HCO3 and Ca < SO4 in 7A and 7B, respectively. Some of these alluvial wells in BDL and CDL have B and F concentrations that are 1 to 5 mg/L, Some of these alluvial wells in BDL and CDL have B and F concentrations that are 1 to 5 mg/L, which is relatively high for fresh to brackish water. Unfortunately, very few alluvial wells were which is relatively high for fresh to brackish water. Unfortunately, very few alluvial wells were analyzed for Li, but those were mostly less than the detection limit (detection limit of 0.1 mg/L). A few analyzed for Li, but those were mostly less than the detection limit (detection limit of 0.1 mg/L). A have concentrations up to 5 mg/L, suggesting that Li, B and F may not be from the same source few have concentrations up to 5 mg/L, suggesting that Li, B and F may not be from the same source because they are not consistently high in the same wells. Boron and fluoride are accessory elements because they are not consistently high in the same wells. Boron and fluoride are accessory elements in igneous rocks and weathering of rock in the mountains surrounding all of the basins is the likely in igneous rocks and weathering of rock in the mountains surrounding all of the basins is the likely source for these elements. The fact that B and F do not concentrate in the brines suggests that they are source for these elements. The fact that B and F do not concentrate in the brines suggests that they adsorbed to the sediment (mostly clays) as the water moves to the playa center, as predicted in arid are adsorbed to the sediment (mostly clays) as the water moves to the playa center, as predicted in zone soils [57]. Although B adsorption is pH dependent, the pH of Bristol Trough brines (around a pH arid zone soils [57]. Although B adsorption is pH dependent, the pH of Bristol Trough brines (around of 6–7) is not low enough to inhibit adsorption [58], and both B and F are likely to adsorb to illite and a pH of 6–7) is not low enough to inhibit adsorption [58], and both B and F are likely to adsorb to montmorillonite clays that are abundant in these basins [59,60]. illite and montmorillonite clays that are abundant in these basins [59,60]. 4.1.2. Brine Chemistry The brine chemistry at BDL has been well documented in numerous publications [7,26,28,34,61], butthTehebbrirnineeccohmepmoisittriyonatoBfDCLDhLasabnedenDwDeLlladreocounmlyendtoecduimnennutmederionu[s6p,8u]b.lNicaetwiondsat[a7,2c6o,l2le8c,3te4d,61in], 2b0u1t7thaendbr2in0e18cofrmomposBiDtioLnaonfdCCDDLLan[1d2,D13D]Laraereconsliystdeonctuwmitehntperdevinio[u6s] andaly[8s]e.sNbeuwt pdraotavicdoellmecoterde inf2o0rm17aatinodn2o0n18thferonmorBthDeLrnanmdaCrgDinLo[1f2B,D13L]arnedcfoonrsCisDteLntbwasitihn-pcerenvteiorubsrianeasly(Fseigsubruetsp6raonvdid8eAm–oDr)e. NinefowrmsamtiopnleosnfrtohmenCoDrtLhewrnermeacroglliencotefdBfDroLmansdevfeoraClDseLtsboafsiwn-eclelsntuesrebdrifnoersC(aFCiglurbersin6eaanndd8Aha–lDit)e. 2 pNreowduscatmionplfersofmrodmepCtDhsLowf 6er0etoco9ll0ecmtebdlsf.roBmristeovlearnaldsCetasdoizf wDreyllsLuakseds aforer NCaC–Cl2ab–rCinl ebraindeshwaliitthe lporwodcuocntcioentfrraotmiondseopfthsuslofaft6e0atnod90almkablilnsi.tBy.riBstDoLl abnrdinCesadcoiznDtariynLmaokresCaraeaNnda–LCiat–hCanl bCrDinLesawndithDlDowL, ecvoenncetnhtoruagtihonthseosfasluinlfiatitesaonfdalallbkrailninesitayr.eBsDimLiblarri.nMesocsotnotfatinhemmoarejoCracaatniodnLsiatnhdanSrCcDorLrealnatdeDwDelLl,inevthene bthrionuegsh(Fthigeusraeli8nAit–ieDs).oNf aollSbrrdinaetas are asivmailarb.leMforostmofthtehealmluavjoiarlcfatnios,nbsuatnbdasSirnc-coernretleartebrwineellsisnhothwe abrsitnreosn(gFcigouvraeri8aAnc–eDb).eNtwoeSerndSartanadreCaava(Filiagbulerefr8oAm).tShoedailulumvianldfaCnsa,cbourtreblastienr-ceelanttievreblyri(nFeisgushreow8Ba) wsterollnegxceopvtarfoiarntcweobevtawlueesnfSrromandBDCLa,(wFihguicrhem8Aa)y. SinodicuamteatnhdatCthaecsoerrtwelaotesarmelpatlievsehlyav(Feigevuorelv8eBd)pwaesltl hexaclietpetsfaotrutrwatoiovna,lukesepfrionmgtBhDeLN,wahaticthmesaayminedcicoantceetnhtartathioensebtuwtoinscarmeapsliensghathveeCvaoclvoendcepnatsrtahtiaolnitse. Ssaotduiruamtioann,dkeKepaipnpgetahretoNhaaavtethtweosapmaethcsoinctehnetrbartionnewbuithinlocrweaesriKnginthseoCmaecsoanmcpenletsrabtiuotnnso.tSodthiuerms and K appear to have two paths in the brine with lower K in some samples but not others (Figure 4.1.2. Brine ChemistryPDF Image | Bristol Dry Lake Brine Compared to Brines from Cadiz
PDF Search Title:
Bristol Dry Lake Brine Compared to Brines from CadizOriginal File Name Searched:
minerals-10-00284-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |