
PDF Publication Title:
Text from PDF Page: 003
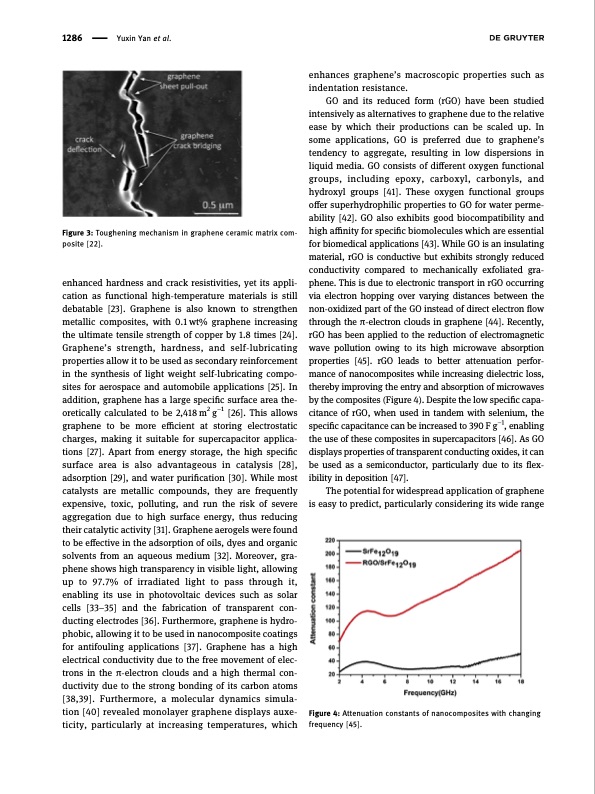
1286 Yuxin Yan et al. Figure 3: Toughening mechanism in graphene ceramic matrix com- posite [22]. enhanced hardness and crack resistivities, yet its appli- cation as functional high-temperature materials is still debatable [23]. Graphene is also known to strengthen metallic composites, with 0.1 wt% graphene increasing the ultimate tensile strength of copper by 1.8 times [24]. Graphene’s strength, hardness, and self-lubricating properties allow it to be used as secondary reinforcement in the synthesis of light weight self-lubricating compo- sites for aerospace and automobile applications [25]. In addition, graphene has a large specific surface area the- oretically calculated to be 2,418 m2 g−1 [26]. This allows graphene to be more efficient at storing electrostatic charges, making it suitable for supercapacitor applica- tions [27]. Apart from energy storage, the high specific surface area is also advantageous in catalysis [28], adsorption [29], and water purification [30]. While most catalysts are metallic compounds, they are frequently expensive, toxic, polluting, and run the risk of severe aggregation due to high surface energy, thus reducing their catalytic activity [31]. Graphene aerogels were found to be effective in the adsorption of oils, dyes and organic solvents from an aqueous medium [32]. Moreover, gra- phene shows high transparency in visible light, allowing up to 97.7% of irradiated light to pass through it, enabling its use in photovoltaic devices such as solar cells [33–35] and the fabrication of transparent con- ducting electrodes [36]. Furthermore, graphene is hydro- phobic, allowing it to be used in nanocomposite coatings for antifouling applications [37]. Graphene has a high electrical conductivity due to the free movement of elec- trons in the π-electron clouds and a high thermal con- ductivity due to the strong bonding of its carbon atoms [38,39]. Furthermore, a molecular dynamics simula- tion [40] revealed monolayer graphene displays auxe- ticity, particularly at increasing temperatures, which enhances graphene’s macroscopic properties such as indentation resistance. GO and its reduced form (rGO) have been studied intensively as alternatives to graphene due to the relative ease by which their productions can be scaled up. In some applications, GO is preferred due to graphene’s tendency to aggregate, resulting in low dispersions in liquid media. GO consists of different oxygen functional groups, including epoxy, carboxyl, carbonyls, and hydroxyl groups [41]. These oxygen functional groups offer superhydrophilic properties to GO for water perme- ability [42]. GO also exhibits good biocompatibility and high affinity for specific biomolecules which are essential for biomedical applications [43]. While GO is an insulating material, rGO is conductive but exhibits strongly reduced conductivity compared to mechanically exfoliated gra- phene. This is due to electronic transport in rGO occurring via electron hopping over varying distances between the non-oxidized part of the GO instead of direct electron flow through the π-electron clouds in graphene [44]. Recently, rGO has been applied to the reduction of electromagnetic wave pollution owing to its high microwave absorption properties [45]. rGO leads to better attenuation perfor- mance of nanocomposites while increasing dielectric loss, thereby improving the entry and absorption of microwaves by the composites (Figure 4). Despite the low specific capa- citance of rGO, when used in tandem with selenium, the specific capacitance can be increased to 390 F g−1, enabling the use of these composites in supercapacitors [46]. As GO displays properties of transparent conducting oxides, it can be used as a semiconductor, particularly due to its flex- ibility in deposition [47]. The potential for widespread application of graphene is easy to predict, particularly considering its wide range Figure 4: Attenuation constants of nanocomposites with changing frequency [45].PDF Image | Synthesis of graphene Potential carbon precursors
PDF Search Title:
Synthesis of graphene Potential carbon precursorsOriginal File Name Searched:
10-1515-ntrev-2020-0100.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |