PDF Publication Title:
Text from PDF Page: 009
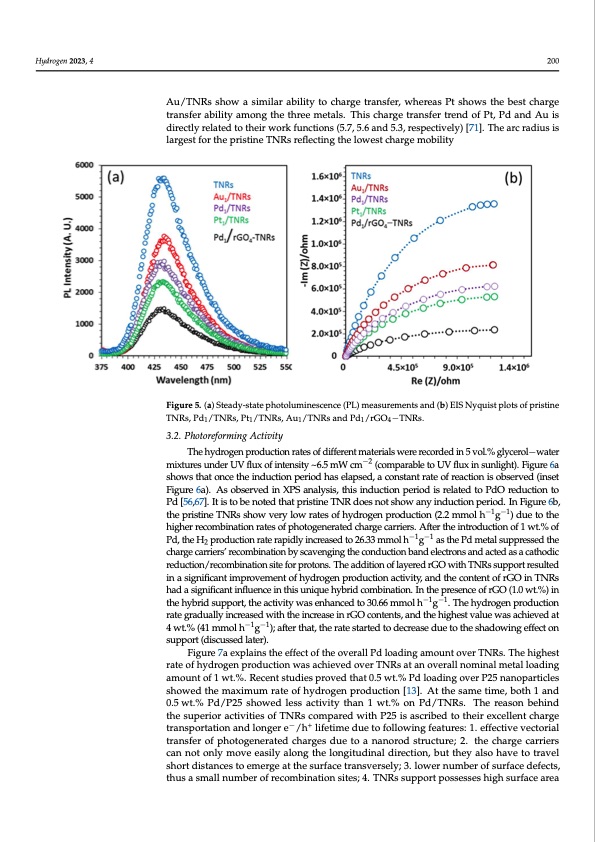
M [70]. Electrochemical impedance spectroscopy (EIS) was performed to study the a a m r s p a o f r g terfacial charge transfer. The Nyquist plots of TNRs, Pd1/TNRs, Pt1/TNRs, Au1/TNRs Pd/rGO4−TNRs are shown in Figure 4b. In general, a smaller arc radius size indic lower charge transfer resistance. Among all materials, Pd/rGO4−TNRs shows the s Hydrogen 2023, 4 200 est arc radius, indicating that it offers the lowest resistance/highest mobility to interfa charge transfer. Pd/TNR and Au/TNRs show a similar ability to charge transfer, whe Pt shows the best charge transfer ability among the three metals. This charge tran Au/TNRs show a similar ability to charge transfer, whereas Pt shows the best charge trend of Pt, Pd and Au is directly related to their work functions (5.7, 5.6 and 5.3, res transfer ability among the three metals. This charge transfer trend of Pt, Pd and Au is tively) [71]. The arc radius is largest for the pristine TNRs reflecting the lowest ch directly related to their work functions (5.7, 5.6 and 5.3, respectively) [71]. The arc radius is moblairlgiteyst for the pristine TNRs reflecting the lowest charge mobility Figure 5. (a) Steady-state photoluminescence (PL) measurements and (b) EIS Nyquist plots of pristine Figure 5. (a) Steady-state photoluminescence (PL) measurements and (b) EIS Nyquist plot TNRs, Pd /TNRs, Pt /TNRs, Au /TNRs and Pd /rGO −TNRs. 11114 pristine TNRs, Pd1/TNRs, Pt1/TNRs, Au1/TNRs and Pd1/rGO4−TNRs. 3.2. Photoreforming Activity 3.2. PhoTtohreehfoydrmroigneng pArocdtuivcititoyn rates of different materials were recorded in 5 vol.% glycerol−water mixtures under UV flux of intensity ~6.5 mW cm−2 (comparable to UV flux in sunlight). Figure 6a The hydrogen production rates of different materials were recorded in 5 v shows that once the induction period has elapsed, a constant rate of reaction is observed (inset glycerol−water mixtures under UV flux of intensity ~6.5 mW cm−2 (comparable to UV Figure 6a). As observed in XPS analysis, this induction period is related to PdO reduction to in sPudn[l5i6g,6h7t])..ItFiisgtoubre n6oatesdhtohawt psrtishtiante oTnNcRedtohees niontdsuhocwtioanypinedruioctdionhapesrieolda.pInseFdig,uarec6ob,nstant the pristine TNRs show very low rates of hydrogen production (2.2 mmol h−1g−1) due to the of reaction is observed (inset Figure 6a). As observed in XPS analysis, this induction riod is related to PdO reduction to Pd [56,67]. It is to be noted that pristine TNR does higher recombination rates of photogenerated charge carriers. After the introduction of 1 wt.% of Pd,theH productionraterapidlyincreasedto26.33mmolh−1g−1asthePdmetalsuppressedthe 2 show any induction period. In Figure 6b, the pristine TNRs show very low rates of charge carriers’ recombination by scavenging the conduction band electrons and acted as a cathodic drogen production (2.2 mmol h−1g−1) due to the higher recombination rates of photo reduction/recombination site for protons. The addition of layered rGO with TNRs support resulted in a significant improvement of hydrogen production activity, and the content of rGO in TNRs had a significant influence in this unique hybrid combination. In the presence of rGO (1.0 wt.%) in the hybrid support, the activity was enhanced to 30.66 mmol h−1g−1. The hydrogen production rate gradually increased with the increase in rGO contents, and the highest value was achieved at 4 wt.% (41 mmol h−1g−1); after that, the rate started to decrease due to the shadowing effect on support (discussed later). Figure 7a explains the effect of the overall Pd loading amount over TNRs. The highest rate of hydrogen production was achieved over TNRs at an overall nominal metal loading amount of 1 wt.%. Recent studies proved that 0.5 wt.% Pd loading over P25 nanoparticles showed the maximum rate of hydrogen production [13]. At the same time, both 1 and 0.5 wt.% Pd/P25 showed less activity than 1 wt.% on Pd/TNRs. The reason behind the superior activities of TNRs compared with P25 is ascribed to their excellent charge transportation and longer e−/h+ lifetime due to following features: 1. effective vectorial transfer of photogenerated charges due to a nanorod structure; 2. the charge carriers can not only move easily along the longitudinal direction, but they also have to travel short distances to emerge at the surface transversely; 3. lower number of surface defects, thus a small number of recombination sites; 4. TNRs support possesses high surface areaPDF Image | Enhanced Photoreforming of Oxygenates
PDF Search Title:
Enhanced Photoreforming of OxygenatesOriginal File Name Searched:
hydrogen-04-00014-v2.pdfDIY PDF Search: Google It | Yahoo | Bing
Salgenx Redox Flow Battery Technology: Power up your energy storage game with Salgenx Salt Water Battery. With its advanced technology, the flow battery provides reliable, scalable, and sustainable energy storage for utility-scale projects. Upgrade to a Salgenx flow battery today and take control of your energy future.
CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com (Standard Web Page)