
PDF Publication Title:
Text from PDF Page: 123
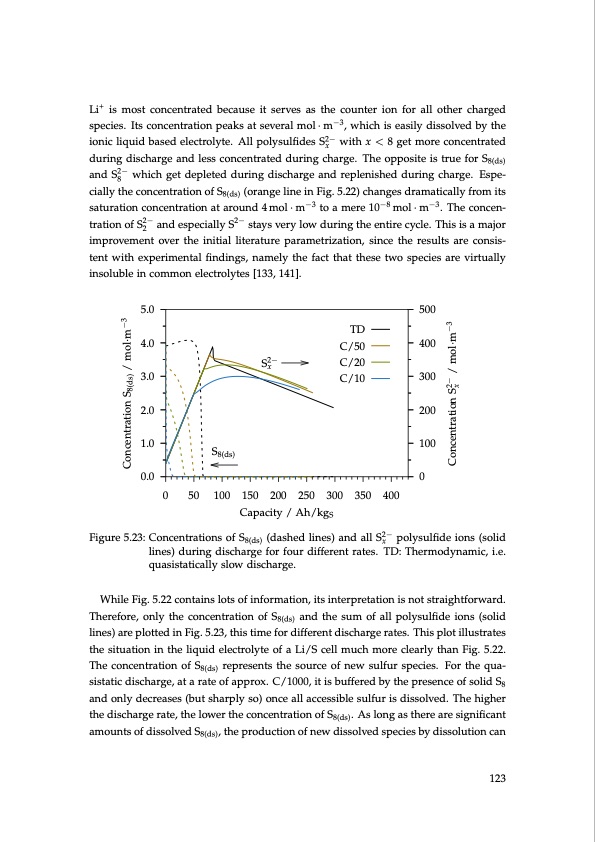
Li+ is most concentrated because it serves as the counter ion for all other charged species. Its concentration peaks at several mol · m−3, which is easily dissolved by the ionic liquid based electrolyte. All polysulfides S2− with x < 8 get more concentrated x during discharge and less concentrated during charge. The opposite is true for S8(ds) and S2− which get depleted during discharge and replenished during charge. Espe- 8 cially the concentration of S8(ds) (orange line in Fig. 5.22) changes dramatically from its saturation concentration at around 4 mol · m−3 to a mere 10−8 mol · m−3. The concen- tration of S2− and especially S2− stays very low during the entire cycle. This is a major 2 improvement over the initial literature parametrization, since the results are consis- tent with experimental findings, namely the fact that these two species are virtually insoluble in common electrolytes [133, 141]. 5.0 500 4.0 400 3.0 300 2.0 200 1.0 100 0.0 0 0 50 100 150 200 250 300 350 400 S8(ds) TD C/50 S2− C/20 x C/10 Capacity / Ah/kgS Figure 5.23: Concentrations of S (dashed lines) and all S2− polysulfide ions (solid 8(ds) x lines) during discharge for four different rates. TD: Thermodynamic, i.e. quasistatically slow discharge. While Fig. 5.22 contains lots of information, its interpretation is not straightforward. Therefore, only the concentration of S8(ds) and the sum of all polysulfide ions (solid lines) are plotted in Fig. 5.23, this time for different discharge rates. This plot illustrates the situation in the liquid electrolyte of a Li/S cell much more clearly than Fig. 5.22. The concentration of S8(ds) represents the source of new sulfur species. For the qua- sistatic discharge, at a rate of approx. C/1000, it is buffered by the presence of solid S8 and only decreases (but sharply so) once all accessible sulfur is dissolved. The higher the discharge rate, the lower the concentration of S8(ds). As long as there are significant amounts of dissolved S8(ds), the production of new dissolved species by dissolution can 123 Concentration S8(ds) / mol·m−3 Concentration S2− / mol·m−3 xPDF Image | Lithium-Sulfur Battery: Design, Characterization, and Physically-based Modeling
PDF Search Title:
Lithium-Sulfur Battery: Design, Characterization, and Physically-based ModelingOriginal File Name Searched:
Dissertation_David_N._Fronczek_The_Lithium_Sulfur_Battery.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |