
PDF Publication Title:
Text from PDF Page: 404
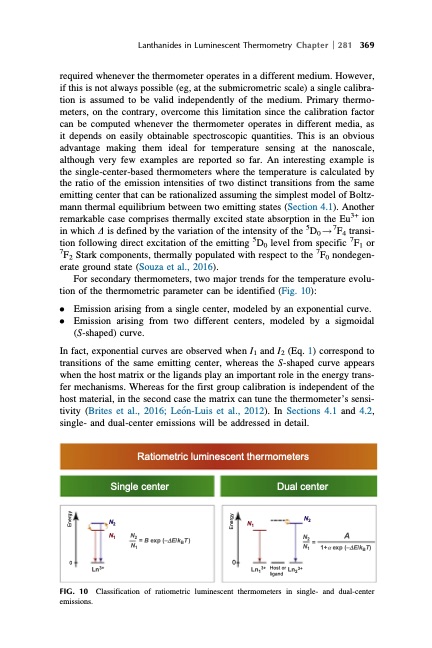
Lanthanides in Luminescent Thermometry Chapter 281 369 required whenever the thermometer operates in a different medium. However, if this is not always possible (eg, at the submicrometric scale) a single calibra- tion is assumed to be valid independently of the medium. Primary thermo- meters, on the contrary, overcome this limitation since the calibration factor can be computed whenever the thermometer operates in different media, as it depends on easily obtainable spectroscopic quantities. This is an obvious advantage making them ideal for temperature sensing at the nanoscale, although very few examples are reported so far. An interesting example is the single-center-based thermometers where the temperature is calculated by the ratio of the emission intensities of two distinct transitions from the same emitting center that can be rationalized assuming the simplest model of Boltz- mann thermal equilibrium between two emitting states (Section 4.1). Another remarkable case comprises thermally excited state absorption in the Eu3+ ion in which D is defined by the variation of the intensity of the 5D0 ! 7F4 transi- tion following direct excitation of the emitting 5D0 level from specific 7F1 or 7F2 Stark components, thermally populated with respect to the 7F0 nondegen- erate ground state (Souza et al., 2016). For secondary thermometers, two major trends for the temperature evolu- tion of the thermometric parameter can be identified (Fig. 10): l Emission arising from a single center, modeled by an exponential curve. l Emission arising from two different centers, modeled by a sigmoidal (S-shaped) curve. In fact, exponential curves are observed when I1 and I2 (Eq. 1) correspond to transitions of the same emitting center, whereas the S-shaped curve appears when the host matrix or the ligands play an important role in the energy trans- fer mechanisms. Whereas for the first group calibration is independent of the host material, in the second case the matrix can tune the thermometer’s sensi- tivity (Brites et al., 2016; Leo ́n-Luis et al., 2012). In Sections 4.1 and 4.2, single- and dual-center emissions will be addressed in detail. Ratiometric luminescent thermometers Single center N2 N1 N2 = B exp (-DE/kBT ) N1 00 Ln3+ Dual center FIG. 10 Classification of ratiometric luminescent thermometers in single- and dual-center emissions. N1 Ln13+ N2 N2 = N1 A 1+a exp (-DE/kBT) Host or Ln23+ ligand Energy EnergyPDF Image | HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHS
PDF Search Title:
HANDBOOK ON THE PHYSICS AND CHEMISTRY OF RARE EARTHSOriginal File Name Searched:
Chemistry-Rare-Earths-49.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |