
PDF Publication Title:
Text from PDF Page: 191
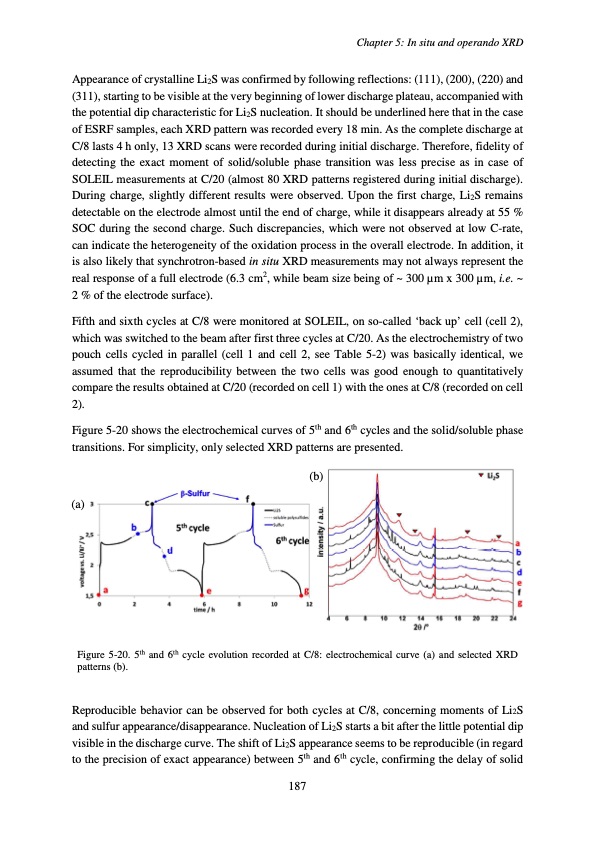
Appearance of crystalline Li2S was confirmed by following reflections: (111), (200), (220) and (311), starting to be visible at the very beginning of lower discharge plateau, accompanied with the potential dip characteristic for Li2S nucleation. It should be underlined here that in the case of ESRF samples, each XRD pattern was recorded every 18 min. As the complete discharge at C/8 lasts 4 h only, 13 XRD scans were recorded during initial discharge. Therefore, fidelity of detecting the exact moment of solid/soluble phase transition was less precise as in case of SOLEIL measurements at C/20 (almost 80 XRD patterns registered during initial discharge). During charge, slightly different results were observed. Upon the first charge, Li2S remains detectable on the electrode almost until the end of charge, while it disappears already at 55 % SOC during the second charge. Such discrepancies, which were not observed at low C-rate, can indicate the heterogeneity of the oxidation process in the overall electrode. In addition, it is also likely that synchrotron-based in situ XRD measurements may not always represent the real response of a full electrode (6.3 cm2, while beam size being of ~ 300 μm x 300 μm, i.e. ~ 2 % of the electrode surface). Fifth and sixth cycles at C/8 were monitored at SOLEIL, on so-called ‘back up’ cell (cell 2), which was switched to the beam after first three cycles at C/20. As the electrochemistry of two pouch cells cycled in parallel (cell 1 and cell 2, see Table 5-2) was basically identical, we assumed that the reproducibility between the two cells was good enough to quantitatively compare the results obtained at C/20 (recorded on cell 1) with the ones at C/8 (recorded on cell 2). Figure 5-20 shows the electrochemical curves of 5th and 6th cycles and the solid/soluble phase transitions. For simplicity, only selected XRD patterns are presented. (b) (a) Chapter 5: In situ and operando XRD Figure 5-20. 5th and 6th cycle evolution recorded at C/8: electrochemical curve (a) and selected XRD patterns (b). Reproducible behavior can be observed for both cycles at C/8, concerning moments of Li2S and sulfur appearance/disappearance. Nucleation of Li2S starts a bit after the little potential dip visible in the discharge curve. The shift of Li2S appearance seems to be reproducible (in regard to the precision of exact appearance) between 5th and 6th cycle, confirming the delay of solid 187PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |