
PDF Publication Title:
Text from PDF Page: 190
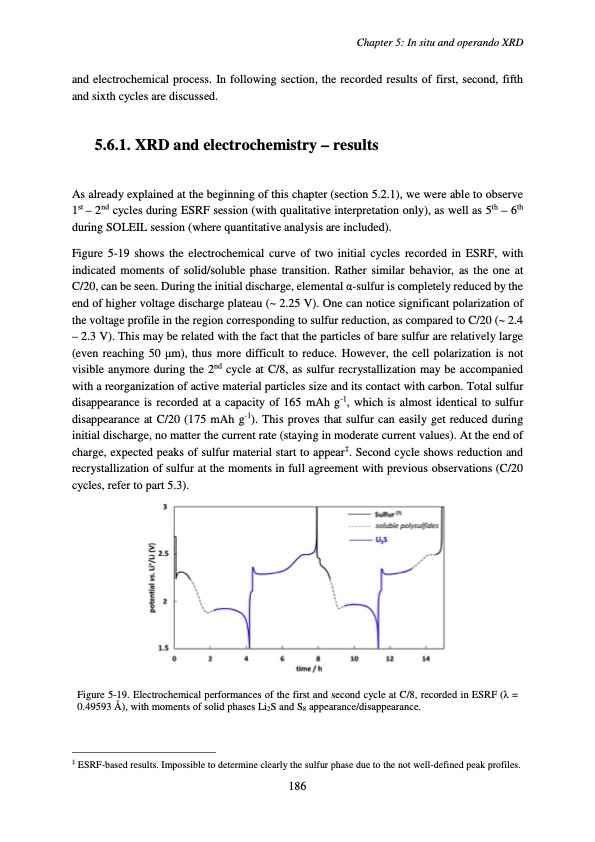
and electrochemical process. In following section, the recorded results of first, second, fifth and sixth cycles are discussed. 5.6.1. XRD and electrochemistry – results As already explained at the beginning of this chapter (section 5.2.1), we were able to observe 1st – 2nd cycles during ESRF session (with qualitative interpretation only), as well as 5th – 6th during SOLEIL session (where quantitative analysis are included). Figure 5-19 shows the electrochemical curve of two initial cycles recorded in ESRF, with indicated moments of solid/soluble phase transition. Rather similar behavior, as the one at C/20, can be seen. During the initial discharge, elemental α-sulfur is completely reduced by the end of higher voltage discharge plateau (~ 2.25 V). One can notice significant polarization of the voltage profile in the region corresponding to sulfur reduction, as compared to C/20 (~ 2.4 – 2.3 V). This may be related with the fact that the particles of bare sulfur are relatively large (even reaching 50 μm), thus more difficult to reduce. However, the cell polarization is not visible anymore during the 2nd cycle at C/8, as sulfur recrystallization may be accompanied with a reorganization of active material particles size and its contact with carbon. Total sulfur disappearance is recorded at a capacity of 165 mAh g-1, which is almost identical to sulfur disappearance at C/20 (175 mAh g-1). This proves that sulfur can easily get reduced during initial discharge, no matter the current rate (staying in moderate current values). At the end of charge, expected peaks of sulfur material start to appear‡. Second cycle shows reduction and recrystallization of sulfur at the moments in full agreement with previous observations (C/20 cycles, refer to part 5.3). Figure 5-19. Electrochemical performances of the first and second cycle at C/8, recorded in ESRF (λ = 0.49593 Å), with moments of solid phases Li2S and S8 appearance/disappearance. ‡ ESRF-based results. Impossible to determine clearly the sulfur phase due to the not well-defined peak profiles. 186 Chapter 5: In situ and operando XRDPDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |