
PDF Publication Title:
Text from PDF Page: 138
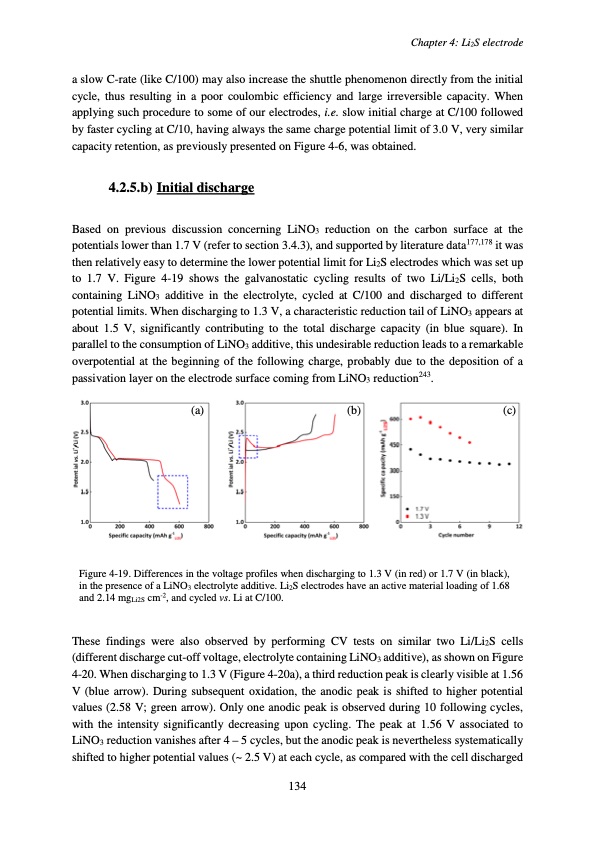
a slow C-rate (like C/100) may also increase the shuttle phenomenon directly from the initial cycle, thus resulting in a poor coulombic efficiency and large irreversible capacity. When applying such procedure to some of our electrodes, i.e. slow initial charge at C/100 followed by faster cycling at C/10, having always the same charge potential limit of 3.0 V, very similar capacity retention, as previously presented on Figure 4-6, was obtained. 4.2.5.b) Initial discharge Based on previous discussion concerning LiNO3 reduction on the carbon surface at the potentials lower than 1.7 V (refer to section 3.4.3), and supported by literature data177,178 it was then relatively easy to determine the lower potential limit for Li2S electrodes which was set up to 1.7 V. Figure 4-19 shows the galvanostatic cycling results of two Li/Li2S cells, both containing LiNO3 additive in the electrolyte, cycled at C/100 and discharged to different potential limits. When discharging to 1.3 V, a characteristic reduction tail of LiNO3 appears at about 1.5 V, significantly contributing to the total discharge capacity (in blue square). In parallel to the consumption of LiNO3 additive, this undesirable reduction leads to a remarkable overpotential at the beginning of the following charge, probably due to the deposition of a passivation layer on the electrode surface coming from LiNO3 reduction243. (a) (b) (c) Figure 4-19. Differences in the voltage profiles when discharging to 1.3 V (in red) or 1.7 V (in black), in the presence of a LiNO3 electrolyte additive. Li2S electrodes have an active material loading of 1.68 and 2.14 mgLi2S cm-2, and cycled vs. Li at C/100. These findings were also observed by performing CV tests on similar two Li/Li2S cells (different discharge cut-off voltage, electrolyte containing LiNO3 additive), as shown on Figure 4-20. When discharging to 1.3 V (Figure 4-20a), a third reduction peak is clearly visible at 1.56 V (blue arrow). During subsequent oxidation, the anodic peak is shifted to higher potential values (2.58 V; green arrow). Only one anodic peak is observed during 10 following cycles, with the intensity significantly decreasing upon cycling. The peak at 1.56 V associated to LiNO3 reduction vanishes after 4 – 5 cycles, but the anodic peak is nevertheless systematically shifted to higher potential values (~ 2.5 V) at each cycle, as compared with the cell discharged Chapter 4: Li2S electrode 134PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |