
PDF Publication Title:
Text from PDF Page: 137
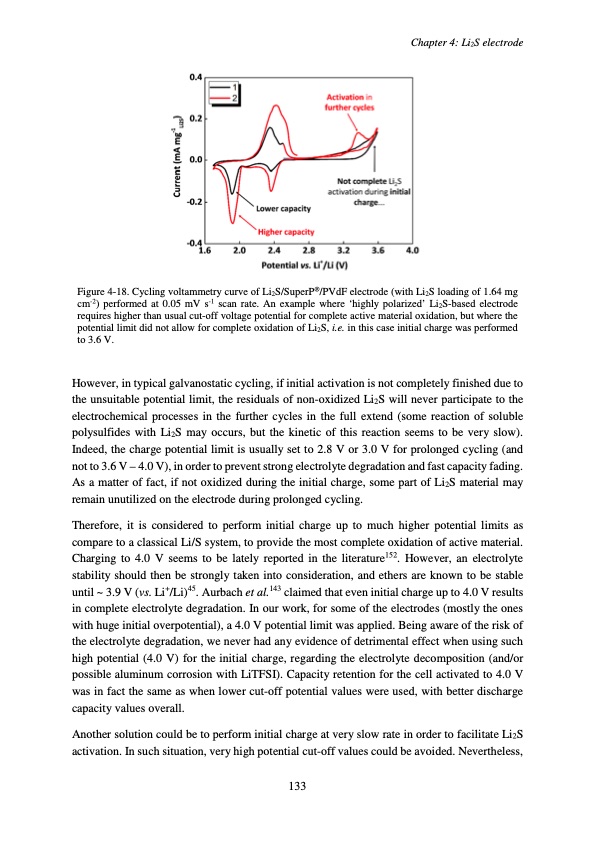
Chapter 4: Li2S electrode Figure 4-18. Cycling voltammetry curve of Li2S/SuperP®/PVdF electrode (with Li2S loading of 1.64 mg cm-2) performed at 0.05 mV s-1 scan rate. An example where ‘highly polarized’ Li2S-based electrode requires higher than usual cut-off voltage potential for complete active material oxidation, but where the potential limit did not allow for complete oxidation of Li2S, i.e. in this case initial charge was performed to 3.6 V. However, in typical galvanostatic cycling, if initial activation is not completely finished due to the unsuitable potential limit, the residuals of non-oxidized Li2S will never participate to the electrochemical processes in the further cycles in the full extend (some reaction of soluble polysulfides with Li2S may occurs, but the kinetic of this reaction seems to be very slow). Indeed, the charge potential limit is usually set to 2.8 V or 3.0 V for prolonged cycling (and not to 3.6 V – 4.0 V), in order to prevent strong electrolyte degradation and fast capacity fading. As a matter of fact, if not oxidized during the initial charge, some part of Li2S material may remain unutilized on the electrode during prolonged cycling. Therefore, it is considered to perform initial charge up to much higher potential limits as compare to a classical Li/S system, to provide the most complete oxidation of active material. Charging to 4.0 V seems to be lately reported in the literature152. However, an electrolyte stability should then be strongly taken into consideration, and ethers are known to be stable until ~ 3.9 V (vs. Li+/Li)45. Aurbach et al.143 claimed that even initial charge up to 4.0 V results in complete electrolyte degradation. In our work, for some of the electrodes (mostly the ones with huge initial overpotential), a 4.0 V potential limit was applied. Being aware of the risk of the electrolyte degradation, we never had any evidence of detrimental effect when using such high potential (4.0 V) for the initial charge, regarding the electrolyte decomposition (and/or possible aluminum corrosion with LiTFSI). Capacity retention for the cell activated to 4.0 V was in fact the same as when lower cut-off potential values were used, with better discharge capacity values overall. Another solution could be to perform initial charge at very slow rate in order to facilitate Li2S activation. In such situation, very high potential cut-off values could be avoided. Nevertheless, 133PDF Image | Accumulateur Lithium Soufre
PDF Search Title:
Accumulateur Lithium SoufreOriginal File Name Searched:
WALUS_2015_archivage.pdfDIY PDF Search: Google It | Yahoo | Bing
Sulfur Deposition on Carbon Nanofibers using Supercritical CO2 Sulfur Deposition on Carbon Nanofibers using Supercritical CO2. Gamma sulfur also known as mother of pearl sulfur and nacreous sulfur... More Info
CO2 Organic Rankine Cycle Experimenter Platform The supercritical CO2 phase change system is both a heat pump and organic rankine cycle which can be used for those purposes and as a supercritical extractor for advanced subcritical and supercritical extraction technology. Uses include producing nanoparticles, precious metal CO2 extraction, lithium battery recycling, and other applications... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |