
PDF Publication Title:
Text from PDF Page: 003
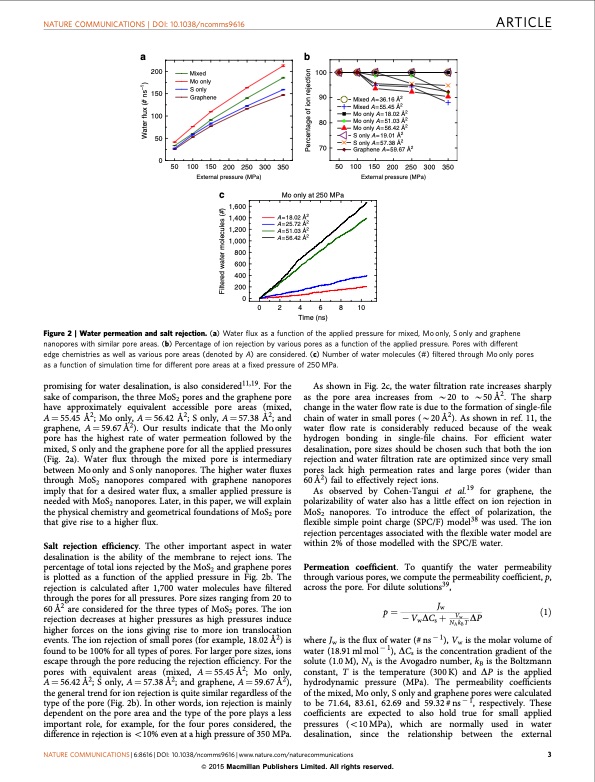
NATURE COMMUNICATIONS | DOI: 10.1038/ncomms9616 ARTICLE ab 200 150 100 50 0 Mixed 100 Mo only S only Graphene 90 Mixed A=36.16 Å2 Mixed A=55.45 Å2 Mo only A=18.02 Å2 Mo only A=51.03 Å2 Mo only A=56.42 Å2 S only A=19.01 Å2 S only A=57.38 Å2 Graphene A=59.67 Å2 50 100 150 200 250 300 350 External pressure (MPa) 50 100 150 200 250 300 350 External pressure (MPa) c Mo only at 250 MPa A=18.02 Å2 A=25.72 Å2 A=51.03 Å2 A=56.42 Å2 80 70 Filtered water molecules (#) Water flux (# ns–1) Percentage of ion rejection promising for water desalination, is also considered11,19. For the sake of comparison, the three MoS2 pores and the graphene pore have approximately equivalent accessible pore areas (mixed, A1⁄455.45 Å2; Mo only, A1⁄456.42 Å2; S only, A1⁄457.38 Å2; and graphene, A 1⁄4 59.67 Å2). Our results indicate that the Mo only pore has the highest rate of water permeation followed by the mixed, S only and the graphene pore for all the applied pressures (Fig. 2a). Water flux through the mixed pore is intermediary between Mo only and S only nanopores. The higher water fluxes through MoS2 nanopores compared with graphene nanopores imply that for a desired water flux, a smaller applied pressure is needed with MoS2 nanopores. Later, in this paper, we will explain the physical chemistry and geometrical foundations of MoS2 pore that give rise to a higher flux. Salt rejection efficiency. The other important aspect in water desalination is the ability of the membrane to reject ions. The percentage of total ions rejected by the MoS2 and graphene pores is plotted as a function of the applied pressure in Fig. 2b. The rejection is calculated after 1,700 water molecules have filtered through the pores for all pressures. Pore sizes ranging from 20 to 60 Å2 are considered for the three types of MoS2 pores. The ion rejection decreases at higher pressures as high pressures induce higher forces on the ions giving rise to more ion translocation events. The ion rejection of small pores (for example, 18.02 Å2) is found to be 100% for all types of pores. For larger pore sizes, ions escape through the pore reducing the rejection efficiency. For the pores with equivalent areas (mixed, A 1⁄4 55.45 Å2; Mo only, A 1⁄4 56.42 Å2; S only, A 1⁄4 57.38 Å2; and graphene, A 1⁄4 59.67 Å2), the general trend for ion rejection is quite similar regardless of the type of the pore (Fig. 2b). In other words, ion rejection is mainly dependent on the pore area and the type of the pore plays a less important role, for example, for the four pores considered, the difference in rejection is o10% even at a high pressure of 350 MPa. As shown in Fig. 2c, the water filtration rate increases sharply as the pore area increases from B20 to B50Å2. The sharp change in the water flow rate is due to the formation of single-file chain of water in small pores (B20 Å2). As shown in ref. 11, the water flow rate is considerably reduced because of the weak hydrogen bonding in single-file chains. For efficient water desalination, pore sizes should be chosen such that both the ion rejection and water filtration rate are optimized since very small pores lack high permeation rates and large pores (wider than 60 Å2) fail to effectively reject ions. As observed by Cohen-Tangui et al.19 for graphene, the polarizability of water also has a little effect on ion rejection in MoS2 nanopores. To introduce the effect of polarization, the flexible simple point charge (SPC/F) model38 was used. The ion rejection percentages associated with the flexible water model are within 2% of those modelled with the SPC/E water. Permeation coefficient. To quantify the water permeability through various pores, we compute the permeability coefficient, p, across the pore. For dilute solutions39, p1⁄4 Jw ð1Þ VwDCsþ Vw DP where Jw is the flux of water (# ns 1), Vw is the molar volume of water (18.91 ml mol 1), DCs is the concentration gradient of the solute (1.0 M), NA is the Avogadro number, kB is the Boltzmann constant, T is the temperature (300K) and DP is the applied hydrodynamic pressure (MPa). The permeability coefficients of the mixed, Mo only, S only and graphene pores were calculated to be 71.64, 83.61, 62.69 and 59.32 # ns 1, respectively. These coefficients are expected to also hold true for small applied pressures (o10MPa), which are normally used in water desalination, since the relationship between the external 1,600 1,400 1,200 1,000 800 600 400 200 0 0 2 4 6 8 10 Time (ns) Figure 2 | Water permeation and salt rejection. (a) Water flux as a function of the applied pressure for mixed, Mo only, S only and graphene nanopores with similar pore areas. (b) Percentage of ion rejection by various pores as a function of the applied pressure. Pores with different edge chemistries as well as various pore areas (denoted by A) are considered. (c) Number of water molecules (#) filtered through Mo only pores as a function of simulation time for different pore areas at a fixed pressure of 250 MPa. NATURE COMMUNICATIONS | 6:8616 | DOI: 10.1038/ncomms9616 | www.nature.com/naturecommunications 3 & 2015 Macmillan Publishers Limited. All rights reserved. NAkBTPDF Image | Water desalination with a single-layer MoS2 nanopore
PDF Search Title:
Water desalination with a single-layer MoS2 nanoporeOriginal File Name Searched:
ncomms9616.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |