
PDF Publication Title:
Text from PDF Page: 004
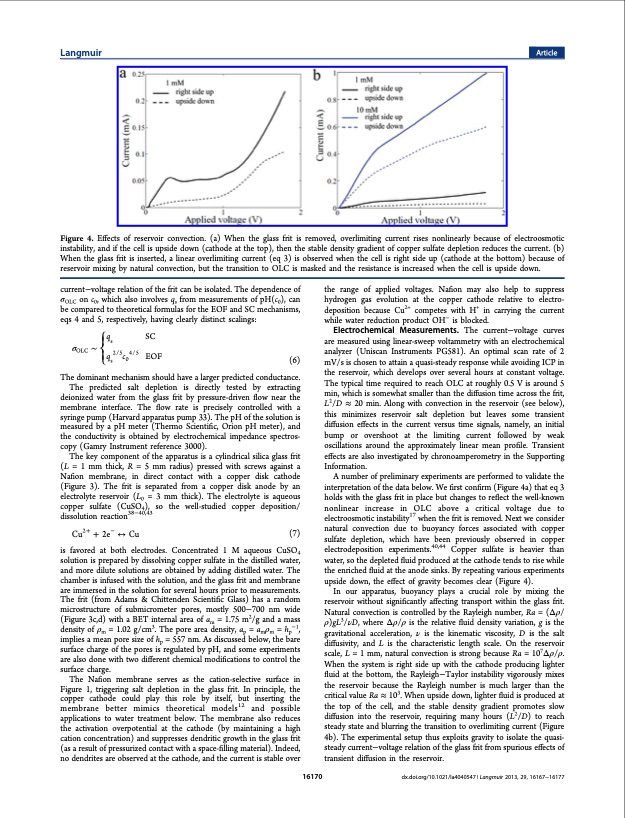
Langmuir Article Figure 4. Effects of reservoir convection. (a) When the glass frit is removed, overlimiting current rises nonlinearly because of electroosmotic instability, and if the cell is upside down (cathode at the top), then the stable density gradient of copper sulfate depletion reduces the current. (b) When the glass frit is inserted, a linear overlimiting current (eq 3) is observed when the cell is right side up (cathode at the bottom) because of reservoir mixing by natural convection, but the transition to OLC is masked and the resistance is increased when the cell is upside down. current−voltage relation of the frit can be isolated. The dependence of σOLC on c0, which also involves qs from measurements of pH(c0), can be compared to theoretical formulas for the EOF and SC mechanisms, eqs 4 and 5, respectively, having clearly distinct scalings: the range of applied voltages. Nafion may also help to suppress hydrogen gas evolution at the copper cathode relative to electro- deposition because Cu2+ competes with H+ in carrying the current while water reduction product OH− is blocked. Electrochemical Measurements. The current−voltage curves are measured using linear-sweep voltammetry with an electrochemical analyzer (Uniscan Instruments PG581). An optimal scan rate of 2 mV/s is chosen to attain a quasi-steady response while avoiding ICP in the reservoir, which develops over several hours at constant voltage. The typical time required to reach OLC at roughly 0.5 V is around 5 min, which is somewhat smaller than the diffusion time across the frit, L2/D ≈ 20 min. Along with convection in the reservoir (see below), this minimizes reservoir salt depletion but leaves some transient diffusion effects in the current versus time signals, namely, an initial bump or overshoot at the limiting current followed by weak oscillations around the approximately linear mean profile. Transient effects are also investigated by chronoamperometry in the Supporting Information. A number of preliminary experiments are performed to validate the interpretation of the data below. We first confirm (Figure 4a) that eq 3 holds with the glass frit in place but changes to reflect the well-known nonlinear increase in OLC above a critical voltage due to electroosmotic instability17 when the frit is removed. Next we consider natural convection due to buoyancy forces associated with copper sulfate depletion, which have been previously observed in copper electrodeposition experiments.40,44 Copper sulfate is heavier than water, so the depleted fluid produced at the cathode tends to rise while the enriched fluid at the anode sinks. By repeating various experiments upside down, the effect of gravity becomes clear (Figure 4). In our apparatus, buoyancy plays a crucial role by mixing the reservoir without significantly affecting transport within the glass frit. Natural convection is controlled by the Rayleigh number, Ra = (Δρ/ ρ)gL3/νD, where Δρ/ρ is the relative fluid density variation, g is the gravitational acceleration, ν is the kinematic viscosity, D is the salt diffusivity, and L is the characteristic length scale. On the reservoir scale, L = 1 mm, natural convection is strong because Ra = 107Δρ/ρ. When the system is right side up with the cathode producing lighter fluid at the bottom, the Rayleigh−Taylor instability vigorously mixes the reservoir because the Rayleigh number is much larger than the critical value Ra ≈ 103. When upside down, lighter fluid is produced at the top of the cell, and the stable density gradient promotes slow diffusion into the reservoir, requiring many hours (L2/D) to reach steady state and blurring the transition to overlimiting current (Figure 4b). The experimental setup thus exploits gravity to isolate the quasi- steady current−voltage relation of the glass frit from spurious effects of transient diffusion in the reservoir. ⎧q SC σ ∼⎪⎨s OLC ⎪q 2/5c 4/5 ⎩s0 (6) EOF The dominant mechanism should have a larger predicted conductance. The predicted salt depletion is directly tested by extracting deionized water from the glass frit by pressure-driven flow near the membrane interface. The flow rate is precisely controlled with a syringe pump (Harvard apparatus pump 33). The pH of the solution is measured by a pH meter (Thermo Scientific, Orion pH meter), and the conductivity is obtained by electrochemical impedance spectros- copy (Gamry Instrument reference 3000). The key component of the apparatus is a cylindrical silica glass frit (L = 1 mm thick, R = 5 mm radius) pressed with screws against a Nafion membrane, in direct contact with a copper disk cathode (Figure 3). The frit is separated from a copper disk anode by an electrolyte reservoir (L0 = 3 mm thick). The electrolyte is aqueous copper sulfate (CuSO4), so the well-studied copper deposition/ dissolution reaction38−40,43 Cu2+ + 2e− ↔ Cu (7) is favored at both electrodes. Concentrated 1 M aqueous CuSO4 solution is prepared by dissolving copper sulfate in the distilled water, and more dilute solutions are obtained by adding distilled water. The chamber is infused with the solution, and the glass frit and membrane are immersed in the solution for several hours prior to measurements. The frit (from Adams & Chittenden Scientific Glass) has a random microstructure of submicrometer pores, mostly 500−700 nm wide (Figure 3c,d) with a BET internal area of am = 1.75 m2/g and a mass density of ρm = 1.02 g/cm3. The pore area density, ap = amρm = hp−1, implies a mean pore size of hp = 557 nm. As discussed below, the bare surface charge of the pores is regulated by pH, and some experiments are also done with two different chemical modifications to control the surface charge. The Nafion membrane serves as the cation-selective surface in Figure 1, triggering salt depletion in the glass frit. In principle, the copper cathode could play this role by itself, but inserting the membrane better mimics theoretical models12 and possible applications to water treatment below. The membrane also reduces the activation overpotential at the cathode (by maintaining a high cation concentration) and suppresses dendritic growth in the glass frit (as a result of pressurized contact with a space-filling material). Indeed, no dendrites are observed at the cathode, and the current is stable over 16170 dx.doi.org/10.1021/la4040547 | Langmuir 2013, 29, 16167−16177PDF Image | Overlimiting Current and Shock Electrodialysis in Porous Media
PDF Search Title:
Overlimiting Current and Shock Electrodialysis in Porous MediaOriginal File Name Searched:
02_Deng_Langmuir2013.pdfDIY PDF Search: Google It | Yahoo | Bing
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
IT XR Project Redstone NFT Available for Sale: NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Be part of the future with this NFT. Can be bought and sold but only one design NFT exists. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Turbine IT XR Project Redstone Design: NFT for sale... NFT for high tech turbine design with one part 3D printed counter-rotating energy turbine. Includes all rights to this turbine design, including license for Fluid Handling Block I and II for the turbine assembly and housing. The NFT includes the blueprints (cad/cam), revenue streams, and all future development of the IT XR Project Redstone... More Info
Infinity Turbine ROT Radial Outflow Turbine 24 Design and Worldwide Rights: NFT for sale... NFT for the ROT 24 energy turbine. Be part of the future with this NFT. This design can be bought and sold but only one design NFT exists. You may manufacture the unit, or get the revenues from its sale from Infinity Turbine. Royalties go to the developer (Infinity) to keep enhancing design and applications... More Info
Infinity Supercritical CO2 10 Liter Extractor Design and Worldwide Rights: The Infinity Supercritical 10L CO2 extractor is for botanical oil extraction, which is rich in terpenes and can produce shelf ready full spectrum oil. With over 5 years of development, this industry leader mature extractor machine has been sold since 2015 and is part of many profitable businesses. The process can also be used for electrowinning, e-waste recycling, and lithium battery recycling, gold mining electronic wastes, precious metals. CO2 can also be used in a reverse fuel cell with nafion to make a gas-to-liquids fuel, such as methanol, ethanol and butanol or ethylene. Supercritical CO2 has also been used for treating nafion to make it more effective catalyst. This NFT is for the purchase of worldwide rights which includes the design. More Info
NFT (Non Fungible Token): Buy our tech, design, development or system NFT and become part of our tech NFT network... More Info
Infinity Turbine Products: Special for this month, any plans are $10,000 for complete Cad/Cam blueprints. License is for one build. Try before you buy a production license. May pay by Bitcoin or other Crypto. Products Page... More Info
| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |