
PDF Publication Title:
Text from PDF Page: 005
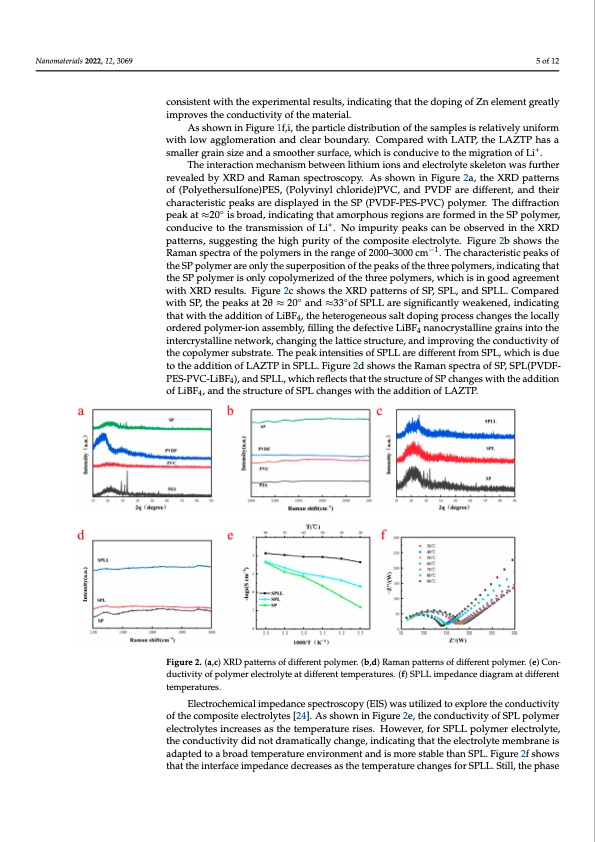
Nanomaterials 2022, 12, 3069 5 of 12 2, x FOR PEER REVIEW consistent with the experimental results, indicating that the doping of Zn element greatly improves the conductivity of the material. As shown in Figure 1f,i, the particle distribution of the samples is relatively uniform with low agglomeration and clear boundary. Compared with LATP, the LAZTP has a smaller grain size and a smoother surface, which is conducive to the migration of Li+. The interaction mechanism between lithium ions and electrolyte skeleton was further revealed by XRD and Raman spectroscopy. As shown in Figure 2a, the XRD patterns of (Polyethersulfone)PES, (Polyvinyl chloride)PVC, and PVDF are different, and their characteristic peaks are displayed in the SP (PVDF-PES-PVC) polymer. The diffraction peak at ≈20◦ is broad, indicating that amorphous regions are formed in the SP polymer, conducive to the transmission of Li+. No impurity peaks can be observed in the XRD patterns, suggesting the high purity of the composite electrolyte. Figure 2b shows the Raman spectra of the polymers in the range of 2000–3000 cm−1. The characteristic peaks of the SP polymer are only the superposition of the peaks of the three polymers, indicating that the SP polymer is only copolymerized of the three polymers, which is in good agreement with XRD results. Figure 2c shows the XRD patterns of SP, SPL, and SPLL. Compared with SP, the peaks at 2θ ≈ 20◦ and ≈33◦of SPLL are significantly weakened, indicating that with the addition of LiBF4, the heterogeneous salt doping process changes the locally ordered polymer-ion assembly, filling the defective LiBF4 nanocrystalline grains into the intercrystalline network, changing the lattice structure, and improving the conductivity of the copolymer substrate. The peak intensities of SPLL are different from SPL, which is due to the addition of LAZTP in SPLL. Figure 2d shows the Raman spectra of SP, SPL(PVDF- PES-PVC-LiBF4), and SPLL, which reflects that the structure of SP changes with the addition of LiBF4, and the structure of SPL changes with the addition of LAZTP. 6 of 13 Figure 2. (a,c) XRD patterns of different polymer. (b,d) Raman patterns of different polymer. (e) Con- ductivity of polymer electrolyte at different temperatures. (f) SPLL impedance diagram at different Figure 2. (a,c) XRD patterns of different polymer. (b,d) Raman patterns of different polymer. (e) temperatures. Conductivity of polymer electrolyte at different temperatures. (f) SPLL impedance diagram at dif- ferent temperatures. Electrochemical impedance spectroscopy (EIS) was utilized to explore the conductivity of the composite electrolytes [24]. As shown in Figure 2e, the conductivity of SPL polymer electrolytes increases as the temperature rises. However, for SPLL polymer electrolyte, Figure 3 shows the preparation flow chart of the polymer electrolyte. PES, PVC, and the conductivity did not dramatically change, indicating that the electrolyte membrane is PVDF polymers constitute a polyelectrolyte and then lithium salt LiBF4 is added to form adapted to a broad temperature environment and is more stable than SPL. Figure 2f shows a stable polymer. LAZTP is used as an additive to improve lithium ion transport channels, that the interface impedance decreases as the temperature changes for SPLL. Still, the phase and inorganic metal oxides are added to increase the amorphous area of the polymer. After mixing uniformly, the composite is poured on a polytetrafluoroethylene template. Subsequently, the composite is cut to the size of a steel sheet after drying. Afterward, the product is assembled into a quasi-solid battery. Then, electrolyte was added to the battery to improve the humidity of the composite interface. The battery was taken out from thePDF Image | Simple Three-Matrix Solid Electrolyte Membrane in Air
PDF Search Title:
Simple Three-Matrix Solid Electrolyte Membrane in AirOriginal File Name Searched:
nanomaterials-12-03069.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |