
PDF Publication Title:
Text from PDF Page: 004
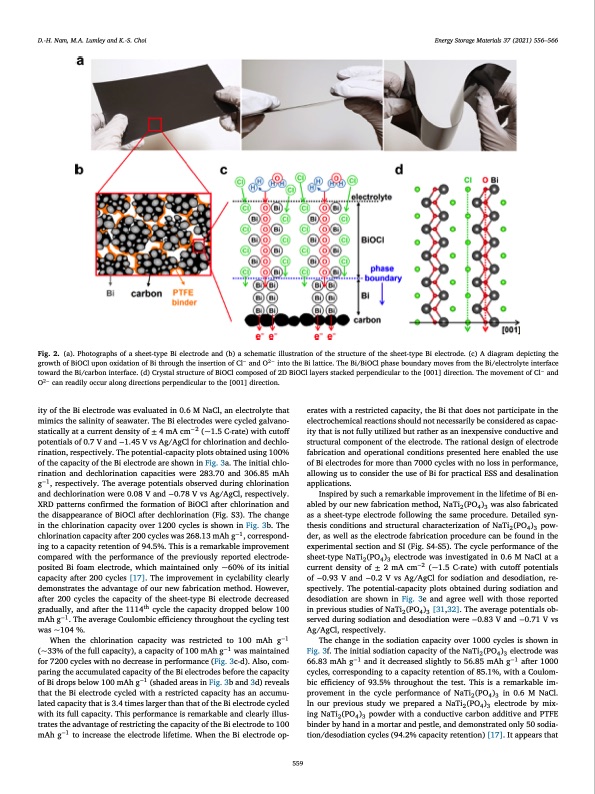
D.-H. Nam, M.A. Lumley and K.-S. Choi Energy Storage Materials 37 (2021) 556–566 Fig. 2. (a). Photographs of a sheet-type Bi electrode and (b) a schematic illustration of the structure of the sheet-type Bi electrode. (c) A diagram depicting the growth of BiOCl upon oxidation of Bi through the insertion of Cl− and O2− into the Bi lattice. The Bi/BiOCl phase boundary moves from the Bi/electrolyte interface toward the Bi/carbon interface. (d) Crystal structure of BiOCl composed of 2D BiOCl layers stacked perpendicular to the [001] direction. The movement of Cl− and O2− can readily occur along directions perpendicular to the [001] direction. ity of the Bi electrode was evaluated in 0.6 M NaCl, an electrolyte that mimics the salinity of seawater. The Bi electrodes were cycled galvano- statically at a current density of ± 4 mA cm−2 (~1.5 C-rate) with cutoff potentials of 0.7 V and −1.45 V vs Ag/AgCl for chlorination and dechlo- rination, respectively. The potential-capacity plots obtained using 100% of the capacity of the Bi electrode are shown in Fig. 3a. The initial chlo- rination and dechlorination capacities were 283.70 and 306.85 mAh g−1, respectively. The average potentials observed during chlorination and dechlorination were 0.08 V and −0.78 V vs Ag/AgCl, respectively. XRD patterns confirmed the formation of BiOCl after chlorination and the disappearance of BiOCl after dechlorination (Fig. S3). The change in the chlorination capacity over 1200 cycles is shown in Fig. 3b. The chlorination capacity after 200 cycles was 268.13 mAh g−1, correspond- ing to a capacity retention of 94.5%. This is a remarkable improvement compared with the performance of the previously reported electrode- posited Bi foam electrode, which maintained only ~60% of its initial capacity after 200 cycles [17]. The improvement in cyclability clearly demonstrates the advantage of our new fabrication method. However, after 200 cycles the capacity of the sheet-type Bi electrode decreased gradually, and after the 1114th cycle the capacity dropped below 100 mAh g−1. The average Coulombic efficiency throughout the cycling test was ~104 %. When the chlorination capacity was restricted to 100 mAh g−1 (~33% of the full capacity), a capacity of 100 mAh g−1 was maintained for 7200 cycles with no decrease in performance (Fig. 3c-d). Also, com- paring the accumulated capacity of the Bi electrodes before the capacity of Bi drops below 100 mAh g−1 (shaded areas in Fig. 3b and 3d) reveals that the Bi electrode cycled with a restricted capacity has an accumu- lated capacity that is 3.4 times larger than that of the Bi electrode cycled with its full capacity. This performance is remarkable and clearly illus- trates the advantage of restricting the capacity of the Bi electrode to 100 mAh g−1 to increase the electrode lifetime. When the Bi electrode op- erates with a restricted capacity, the Bi that does not participate in the electrochemical reactions should not necessarily be considered as capac- ity that is not fully utilized but rather as an inexpensive conductive and structural component of the electrode. The rational design of electrode fabrication and operational conditions presented here enabled the use of Bi electrodes for more than 7000 cycles with no loss in performance, allowing us to consider the use of Bi for practical ESS and desalination applications. Inspired by such a remarkable improvement in the lifetime of Bi en- abled by our new fabrication method, NaTi2 (PO4 )3 was also fabricated as a sheet-type electrode following the same procedure. Detailed syn- thesis conditions and structural characterization of NaTi2 (PO4 )3 pow- der, as well as the electrode fabrication procedure can be found in the experimental section and SI (Fig. S4-S5). The cycle performance of the sheet-type NaTi2 (PO4 )3 electrode was investigated in 0.6 M NaCl at a current density of ± 2 mA cm−2 (~1.5 C-rate) with cutoff potentials of −0.93 V and −0.2 V vs Ag/AgCl for sodiation and desodiation, re- spectively. The potential-capacity plots obtained during sodiation and desodiation are shown in Fig. 3e and agree well with those reported in previous studies of NaTi2 (PO4 )3 [31,32]. The average potentials ob- served during sodiation and desodiation were −0.83 V and −0.71 V vs Ag/AgCl, respectively. The change in the sodiation capacity over 1000 cycles is shown in Fig. 3f. The initial sodiation capacity of the NaTi2 (PO4 )3 electrode was 66.83 mAh g−1 and it decreased slightly to 56.85 mAh g−1 after 1000 cycles, corresponding to a capacity retention of 85.1%, with a Coulom- bic efficiency of 93.5% throughout the test. This is a remarkable im- provement in the cycle performance of NaTi2 (PO4 )3 in 0.6 M NaCl. In our previous study we prepared a NaTi2 (PO4 )3 electrode by mix- ing NaTi2 (PO4 )3 powder with a conductive carbon additive and PTFE binder by hand in a mortar and pestle, and demonstrated only 50 sodia- tion/desodiation cycles (94.2% capacity retention) [17]. It appears that 559PDF Image | seawater battery with desalination capabilities
PDF Search Title:
seawater battery with desalination capabilitiesOriginal File Name Searched:
10279292.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |