
PDF Publication Title:
Text from PDF Page: 052
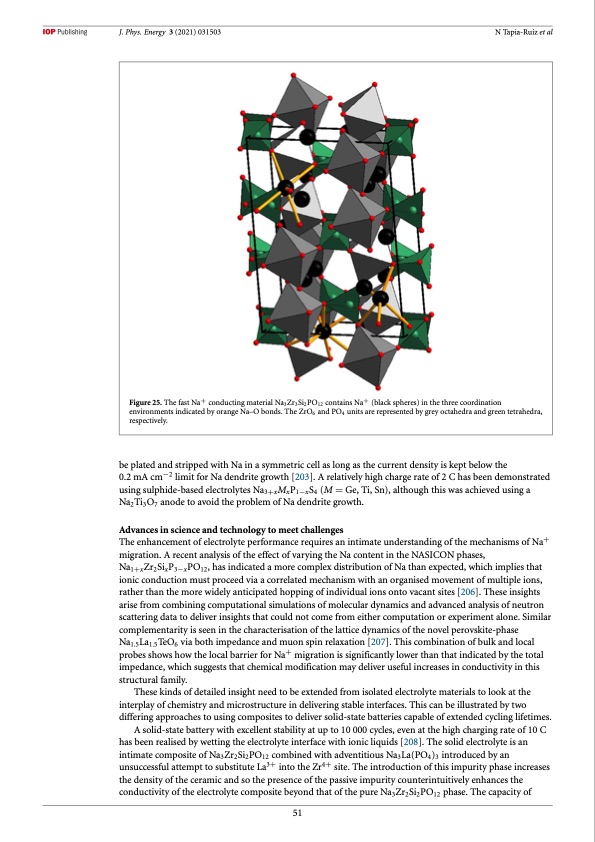
J. Phys. Energy 3 (2021) 031503 N Tapia-Ruiz et al Figure 25. The fast Na+ conducting material Na3Zr3Si2PO12 contains Na+ (black spheres) in the three coordination environments indicated by orange Na–O bonds. The ZrO6 and PO4 units are represented by grey octahedra and green tetrahedra, respectively. be plated and stripped with Na in a symmetric cell as long as the current density is kept below the 0.2 mA cm−2 limit for Na dendrite growth [203]. A relatively high charge rate of 2 C has been demonstrated using sulphide-based electrolytes Na3+xMxP1−xS4 (M = Ge, Ti, Sn), although this was achieved using a Na2Ti3O7 anode to avoid the problem of Na dendrite growth. Advances in science and technology to meet challenges The enhancement of electrolyte performance requires an intimate understanding of the mechanisms of Na+ migration. A recent analysis of the effect of varying the Na content in the NASICON phases, Na1+xZr2SixP3−xPO12, has indicated a more complex distribution of Na than expected, which implies that ionic conduction must proceed via a correlated mechanism with an organised movement of multiple ions, rather than the more widely anticipated hopping of individual ions onto vacant sites [206]. These insights arise from combining computational simulations of molecular dynamics and advanced analysis of neutron scattering data to deliver insights that could not come from either computation or experiment alone. Similar complementarity is seen in the characterisation of the lattice dynamics of the novel perovskite-phase Na1.5La1.5TeO6 via both impedance and muon spin relaxation [207]. This combination of bulk and local probes shows how the local barrier for Na+ migration is significantly lower than that indicated by the total impedance, which suggests that chemical modification may deliver useful increases in conductivity in this structural family. These kinds of detailed insight need to be extended from isolated electrolyte materials to look at the interplay of chemistry and microstructure in delivering stable interfaces. This can be illustrated by two differing approaches to using composites to deliver solid-state batteries capable of extended cycling lifetimes. A solid-state battery with excellent stability at up to 10 000 cycles, even at the high charging rate of 10 C has been realised by wetting the electrolyte interface with ionic liquids [208]. The solid electrolyte is an intimate composite of Na3Zr2Si2PO12 combined with adventitious Na3La(PO4)3 introduced by an unsuccessful attempt to substitute La3+ into the Zr4+ site. The introduction of this impurity phase increases the density of the ceramic and so the presence of the passive impurity counterintuitively enhances the conductivity of the electrolyte composite beyond that of the pure Na3Zr2Si2PO12 phase. The capacity of 51PDF Image | roadmap for sodium-ion batteries
PDF Search Title:
roadmap for sodium-ion batteriesOriginal File Name Searched:
sodium-ion-batteries.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |