
PDF Publication Title:
Text from PDF Page: 030
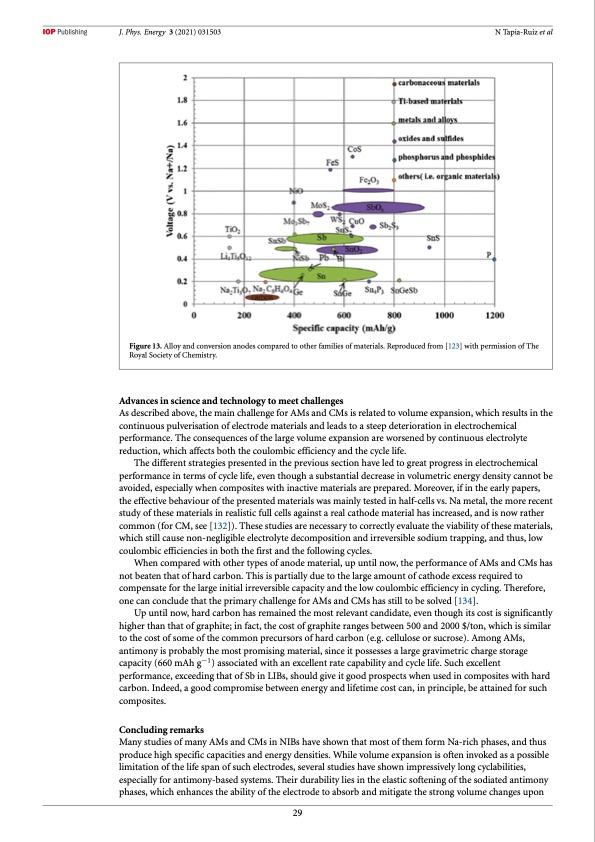
J. Phys. Energy 3 (2021) 031503 N Tapia-Ruiz et al Figure 13. Alloy and conversion anodes compared to other families of materials. Reproduced from [123] with permission of The Royal Society of Chemistry. Advances in science and technology to meet challenges As described above, the main challenge for AMs and CMs is related to volume expansion, which results in the continuous pulverisation of electrode materials and leads to a steep deterioration in electrochemical performance. The consequences of the large volume expansion are worsened by continuous electrolyte reduction, which affects both the coulombic efficiency and the cycle life. The different strategies presented in the previous section have led to great progress in electrochemical performance in terms of cycle life, even though a substantial decrease in volumetric energy density cannot be avoided, especially when composites with inactive materials are prepared. Moreover, if in the early papers, the effective behaviour of the presented materials was mainly tested in half-cells vs. Na metal, the more recent study of these materials in realistic full cells against a real cathode material has increased, and is now rather common (for CM, see [132]). These studies are necessary to correctly evaluate the viability of these materials, which still cause non-negligible electrolyte decomposition and irreversible sodium trapping, and thus, low coulombic efficiencies in both the first and the following cycles. When compared with other types of anode material, up until now, the performance of AMs and CMs has not beaten that of hard carbon. This is partially due to the large amount of cathode excess required to compensate for the large initial irreversible capacity and the low coulombic efficiency in cycling. Therefore, one can conclude that the primary challenge for AMs and CMs has still to be solved [134]. Up until now, hard carbon has remained the most relevant candidate, even though its cost is significantly higher than that of graphite; in fact, the cost of graphite ranges between 500 and 2000 $/ton, which is similar to the cost of some of the common precursors of hard carbon (e.g. cellulose or sucrose). Among AMs, antimony is probably the most promising material, since it possesses a large gravimetric charge storage capacity (660 mAh g−1) associated with an excellent rate capability and cycle life. Such excellent performance, exceeding that of Sb in LIBs, should give it good prospects when used in composites with hard carbon. Indeed, a good compromise between energy and lifetime cost can, in principle, be attained for such composites. Concluding remarks Many studies of many AMs and CMs in NIBs have shown that most of them form Na-rich phases, and thus produce high specific capacities and energy densities. While volume expansion is often invoked as a possible limitation of the life span of such electrodes, several studies have shown impressively long cyclabilities, especially for antimony-based systems. Their durability lies in the elastic softening of the sodiated antimony phases, which enhances the ability of the electrode to absorb and mitigate the strong volume changes upon 29PDF Image | roadmap for sodium-ion batteries
PDF Search Title:
roadmap for sodium-ion batteriesOriginal File Name Searched:
sodium-ion-batteries.pdfDIY PDF Search: Google It | Yahoo | Bing
Product and Development Focus for Infinity Turbine
ORC Waste Heat Turbine and ORC System Build Plans: All turbine plans are $10,000 each. This allows you to build a system and then consider licensing for production after you have completed and tested a unit.Redox Flow Battery Technology: With the advent of the new USA tax credits for producing and selling batteries ($35/kW) we are focussing on a simple flow battery using shipping containers as the modular electrolyte storage units with tax credits up to $140,000 per system. Our main focus is on the salt battery. This battery can be used for both thermal and electrical storage applications. We call it the Cogeneration Battery or Cogen Battery. One project is converting salt (brine) based water conditioners to simultaneously produce power. In addition, there are many opportunities to extract Lithium from brine (salt lakes, groundwater, and producer water).Salt water or brine are huge sources for lithium. Most of the worlds lithium is acquired from a brine source. It's even in seawater in a low concentration. Brine is also a byproduct of huge powerplants, which can now use that as an electrolyte and a huge flow battery (which allows storage at the source).We welcome any business and equipment inquiries, as well as licensing our turbines for manufacturing.| CONTACT TEL: 608-238-6001 Email: greg@infinityturbine.com | RSS | AMP |